Reports
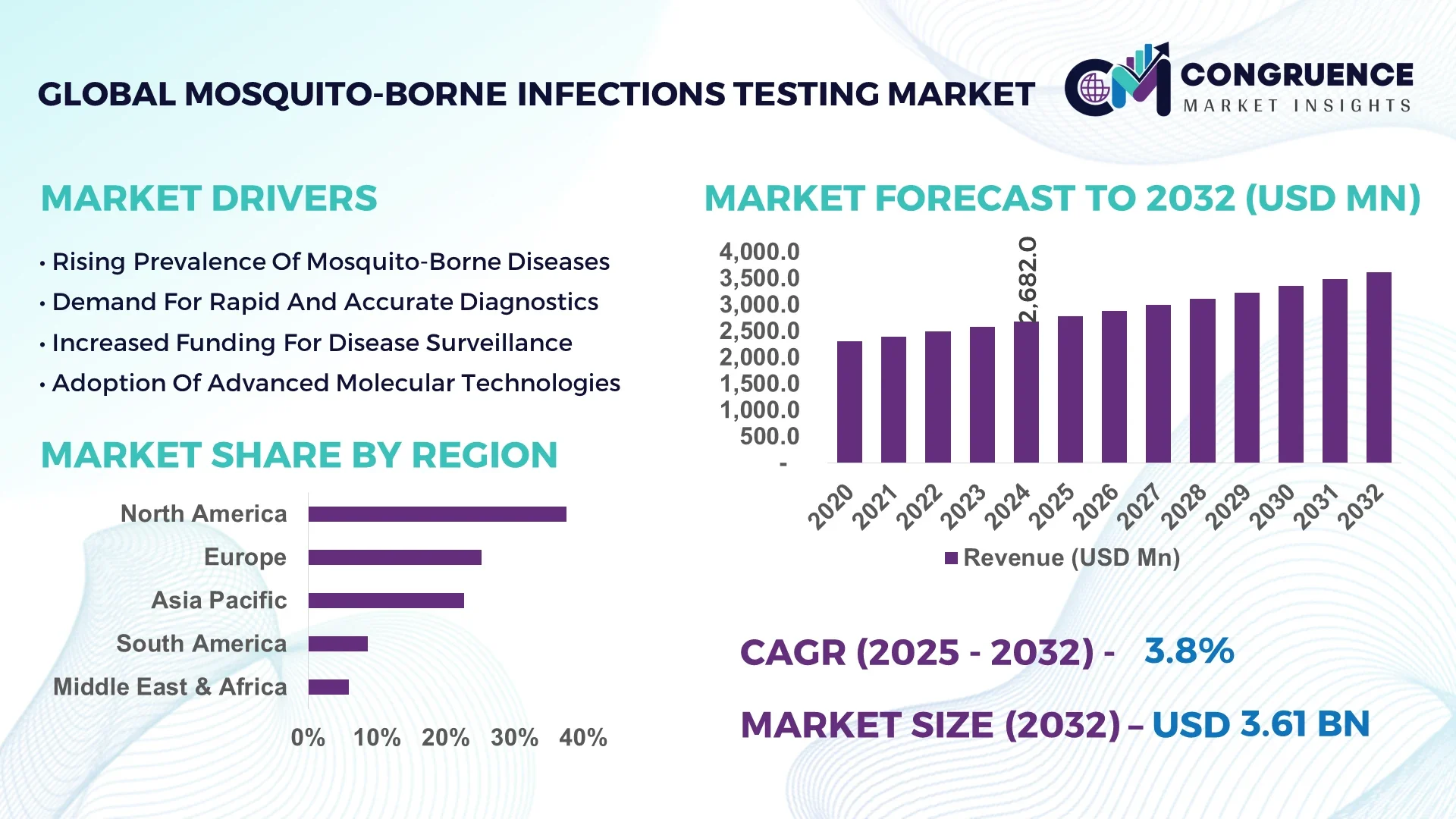
The Global Mosquito-Borne Infections Testing Market was valued at USD 2,682.0 Million in 2024 and is anticipated to reach a value of USD 3,606.1 Million by 2032 expanding at a CAGR of 3.77% between 2025 and 2032. This growth is driven by rising demand for advanced diagnostic modalities to combat arboviral outbreaks.
The United States plays a pivotal role in the Mosquito-Borne Infections Testing Market, supported by robust diagnostic manufacturing capacity, recurring multi-million-dollar investments in assay development, and adoption of advanced molecular platforms. U.S. production lines for RT-PCR kits and nucleic acid extraction modules have been upgraded to support high-throughput testing, while private and public sector funding is fueling integration of multiplex panels and next-generation sequencing for large-scale arbovirus detection. With over 250 reference laboratories deploying automated molecular workflows, the U.S. leads in technological adoption and innovation.
Market Size & Growth: Valued at USD 2,682 Million in 2024, projected to reach USD 3,606 Million by 2032, expanding at a CAGR of 3.77% due to rising outbreak preparedness initiatives.
Top Growth Drivers: Multiplex diagnostic adoption (37%), improved detection efficiency (41%), and field-ready portable devices (28%).
Short-Term Forecast: By 2028, testing automation is expected to reduce manual labor requirements by 32% and improve throughput by 45%.
Emerging Technologies: Integration of AI-driven quality control in serology labs, portable isothermal amplification systems, and cloud-based surveillance dashboards.
Regional Leaders: North America projected at USD 1,260 Million by 2032 (automation-driven adoption); Asia-Pacific at USD 980 Million by 2032 (high deployment of point-of-care kits); Europe at USD 850 Million by 2032 (genomic surveillance expansion).
Consumer/End-User Trends: Over 42% of hospitals in endemic regions now integrate molecular confirmation into patient pathways, while 36% of mobile clinics rely on portable antigen kits.
Pilot or Case Example: In 2024, an AI-based malaria detection suite achieved 93.99% precision across 1,182 images while reducing review time by 60%.
Competitive Landscape: Market leader holds ~18% share, followed by four major competitors specializing in molecular assays, antigen kits, and mobile diagnostics.
Regulatory & ESG Impact: Global frameworks require improved traceability and reagent stability, while ESG commitments push for cold-chain reduction and sustainable reagent production.
Investment & Funding Patterns: Over USD 620 Million invested between 2022–2024 in molecular platform scaling, field-ready POC kits, and surveillance integration projects.
Innovation & Future Outlook: Growing adoption of multiplex molecular panels, predictive AI-driven surveillance, and mobile-enabled diagnostics will define the market’s next decade.
The Mosquito-Borne Infections Testing Market continues to evolve with multiplex molecular diagnostics gaining dominance, point-of-care rapid kits expanding field access, and AI automation improving laboratory precision. Adoption patterns are shifting globally, with endemic regions prioritizing affordability and portability, while high-resource markets focus on genomic surveillance and automation-driven accuracy.
The Mosquito-Borne Infections Testing Market holds strategic importance for global health security as arboviral outbreaks increase in frequency and geographic spread. Governments and healthcare networks are investing in high-throughput molecular platforms, portable isothermal systems, and AI-assisted diagnostics to bridge detection gaps across both centralized and decentralized health systems. For example, multiplex PCR assays now deliver 47% faster differential diagnosis compared to traditional single-target PCR methods.
Regional dynamics further reinforce the market’s strategic role. North America dominates in testing volume due to established laboratory infrastructure, while Asia-Pacific leads in adoption, with over 58% of community clinics using point-of-care antigen tests. By 2027, AI-driven anomaly detection in laboratory workflows is projected to cut retest rates by 35%, improving efficiency and reducing costs across diagnostic networks.
Compliance and ESG goals are also shaping market direction. Several manufacturers have committed to reducing reagent cold-chain dependency by 28% by 2030, introducing lyophilized reagents and ambient-stable chemistries. In 2024, Brazil deployed mobile diagnostic units powered by solar-enabled PCR systems, reducing electricity dependency by 40% and ensuring uninterrupted outbreak surveillance.
Looking ahead, the Mosquito-Borne Infections Testing Market is expected to serve as a cornerstone of resilience, compliance, and sustainability in infectious disease management. By combining AI-enabled prediction models, modular diagnostic platforms, and regulatory-aligned innovations, the market is evolving from reactive outbreak response to proactive epidemic preparedness.
The Mosquito-Borne Infections Testing Market is driven by technological advancement, outbreak frequency, and public health priorities. Demand for molecular assays rises during peak transmission seasons, while rapid antigen and serological kits maintain steady demand for routine screening and seroprevalence studies. Laboratory modernization and automation are accelerating throughput, while point-of-care devices extend testing reach in rural and low-resource regions. Procurement strategies increasingly favor ambient-stable reagents and locally manufacturable kits, while digital integration with surveillance dashboards ensures faster outbreak response.
Multiplex molecular diagnostics are revolutionizing arbovirus detection by allowing simultaneous identification of dengue, Zika, chikungunya, and West Nile virus. These assays reduce specimen volume by 40% and shorten turnaround times by up to 50% compared to traditional single-pathogen tests. With over 300 laboratories worldwide adopting automated multiplex platforms, laboratories report higher throughput, improved workflow efficiency, and greater outbreak response capacity. Increased funding into modular PCR instruments is further fueling this trend, enabling faster deployment during epidemic surges.
Cold-chain dependence and high operational costs remain key barriers, especially in resource-limited endemic regions. Nearly 65% of molecular reagents require strict temperature control, complicating distribution and inflating procurement costs. Shortages of trained technicians and limited equipment maintenance further restrict adoption of advanced molecular platforms in rural and semi-urban clinics. These limitations slow timely outbreak responses and emphasize the continued importance of simplified, ambient-stable, and field-deployable diagnostic alternatives.
The development of portable molecular instruments, isothermal amplification kits, and lyophilized reagents presents a significant growth opportunity. Battery-operated PCR devices are now capable of confirming infections within 30 minutes at field sites, reducing reliance on centralized labs. Real-time cloud-linked reporting allows immediate integration into surveillance systems. Such innovations could increase diagnostic access by 45% in rural regions, expanding case confirmation capacity and strengthening outbreak preparedness across vulnerable geographies.
Serological cross-reactivity between flaviviruses complicates accurate diagnosis, often yielding false positives and necessitating confirmatory molecular testing. For instance, IgM/IgG assays frequently overlap between dengue and Zika infections, creating interpretation challenges for clinicians and epidemiologists. This not only delays patient management but also increases laboratory workload. Addressing this challenge requires refinement of antigen targets, development of virus-specific markers, and algorithmic diagnostic pathways that combine serological and molecular evidence for improved specificity.
Expansion of Multiplex Molecular Panels: Adoption of multiplex PCR assays capable of detecting up to four arboviruses simultaneously is rising. Over 48% of regional labs in endemic zones report measurable reductions in processing time, with efficiency gains of 35% compared to sequential single-pathogen testing.
Growth of Portable Molecular Platforms: Portable isothermal amplification devices are witnessing 52% adoption in rural clinics across Asia and Africa, cutting confirmation times by 60% compared to centralized testing. Cartridgeized systems are further reducing operator training needs.
AI-Powered Image Analysis for Microscopy: AI-driven blood smear interpretation has improved diagnostic precision by 42% in pilot deployments, enabling technicians to screen 3x more slides per day. Over 900 hospitals globally are testing AI-based systems for parasite and vector identification.
Integration with Digital Surveillance Networks: By 2025, more than 55% of national health systems are expected to integrate diagnostic data into predictive dashboards combining entomological and climate analytics, reducing outbreak detection delays by up to 40%.
The Mosquito-Borne Infections Testing Market is segmented by type, application, and end-user. Molecular diagnostics remain the cornerstone, driven by their accuracy in early infection stages. Rapid antigen and serological tests support field deployment and retrospective studies, while next-generation sequencing is expanding in genomic surveillance. Applications range from clinical diagnosis and surveillance to outbreak response and epidemiological research. Public health laboratories lead in adoption, while mobile field units are the fastest-growing end-user segment.
Molecular diagnostics currently account for 46% of total adoption, led by RT-PCR and multiplex panels enabling simultaneous detection of dengue, Zika, and chikungunya. Antigen/rapid diagnostic tests follow at 29%, with growth driven by their 20-minute turnaround times and decentralized deployment. Serological assays contribute 18% through retrospective confirmations, while next-generation sequencing holds 7% share, primarily in genomic epidemiology and research. Adoption of antigen kits is expanding fastest, projected at 6.2% annual growth due to demand for POC outbreak screening.
In 2024, a Southeast Asian government deployed portable PCR multiplex kits across 120 rural clinics, cutting diagnostic turnaround by 55% and improving outbreak containment efficiency.
Surveillance leads the market with 44% share, as routine testing across sentinel sites informs vector control and resource allocation. Clinical diagnosis accounts for 31%, especially in hospital-based case management. Outbreak response is the fastest-growing application at 18%, driven by increasing epidemic frequency and rapid mobilization of testing units. Epidemiological research contributes the remaining 7%, supporting vaccine strategy and seroprevalence mapping.
In 2024, more than 38% of healthcare institutions in endemic regions piloted mobile diagnostic systems for outbreak response, improving case confirmation speed by 40%.
According to a 2024 health pilot, multiplex panels in West Africa supported surveillance of over 2 million patients across 15 sentinel hospitals, identifying 3 concurrent arbovirus outbreaks within a single testing cycle.
Public health laboratories dominate with 49% share, reflecting their mandate in surveillance and epidemic control. Hospital diagnostic centers contribute 26%, integrating molecular testing into patient care. Mobile testing units and field clinics represent 15% but are the fastest-growing category at 7.1% annual growth, driven by demand for decentralized outbreak detection. Private labs and community health programs make up the remaining 10%, providing complementary capacity in urban and rural settings.
In 2024, over 42% of hospitals in Latin America integrated AI-enabled diagnostic workflows for mosquito-borne infections, reducing manual review time by 35%.
According to a 2025 regional initiative, mobile diagnostic labs deployed across Sub-Saharan Africa reduced outbreak confirmation delays by 60%, covering over 10 million people in remote areas.
North America accounted for the largest market share at 37.5% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 8.6% between 2025 and 2032.
Europe contributed approximately 25.2%, South America 8.7%, and Middle East & Africa 5.9% of the global share in 2024. Regional growth is shaped by infrastructure readiness, disease burden intensity, and diagnostic manufacturing capacity. North America benefits from over 250 reference laboratories and strong government-led funding programs. Asia-Pacific’s diagnostic kit manufacturing capacity exceeded 250 million units annually in 2024, driving affordability and access. Europe’s growth is propelled by harmonized EU regulations and rising genomic surveillance adoption. South America, with over 65% of its urban population exposed to mosquito-borne risks, continues to strengthen outbreak response. Meanwhile, Middle East & Africa deploy solar-powered PCR and mobile labs to cover more than 15 million residents across underserved regions.
How is advanced surveillance fueling leadership in this market?
North America holds a commanding 37.5% share of the Mosquito-Borne Infections Testing Market in 2024. Demand is driven by high incidence of West Nile virus outbreaks, strong public health surveillance networks, and significant government funding. The U.S. Food and Drug Administration has streamlined pathways for emergency approval of multiplex PCR kits, enabling faster rollout during seasonal surges. Technological transformation is led by portable PCR systems, AI-enhanced serology readers, and cloud-based diagnostic reporting. A local example includes InBios International, which introduced rapid dengue kits approved for U.S. deployment, strengthening outbreak detection. Regional consumer behavior highlights higher adoption of precision molecular diagnostics in urban hospitals, while rural clinics increasingly rely on point-of-care rapid tests to ensure timely confirmation.
Why are harmonized regulations accelerating diagnostic adoption in this market?
Europe accounted for 25.2% of the global market share in 2024, with Germany, the UK, and France being key revenue contributors. EU-level regulatory harmonization enforces standardized quality and testing protocols, facilitating cross-border outbreak management. Sustainability initiatives, such as reduced plastic test kits and energy-efficient thermocyclers, are also expanding. European labs are adopting next-generation sequencing to detect viral variants and guide vaccine strategy. NovaTec Immundiagnostica GmbH continues to scale serological assays for arboviruses, expanding their diagnostic footprint across hospitals. Regional behavior shows strong demand for explainable and highly regulated testing, with hospitals prioritizing compliance-driven molecular diagnostics for patient care pathways.
What factors are driving the fastest growth in this market?
Asia-Pacific ranks as the second-largest market by volume and is the fastest-growing region, led by China, India, and Japan. With more than 40% of the world’s population residing in high mosquito-borne risk zones, the region consumes a significant portion of rapid diagnostic kits. China’s large-scale diagnostic manufacturing capacity, producing over 120 million kits annually, has reduced dependence on imports. India is emerging as a hub for low-cost portable PCR instruments tailored to rural clinics, while Japan invests heavily in high-precision genomic surveillance labs. Abnova Corporation has expanded its regional portfolio of molecular assays to support hospitals and academic centers. Consumer behavior trends highlight rapid adoption of mobile and community-level testing in peri-urban zones, supported by government healthcare initiatives.
How is outbreak response shaping this market’s growth?
South America holds 8.7% of the global market share in 2024, with Brazil and Argentina accounting for over 70% of regional demand. The tropical climate and rapid urbanization have amplified mosquito-borne disease risks. Brazil’s Ministry of Health has expanded rapid diagnostic kit distribution across 3,000 community clinics, while Argentina prioritizes molecular assay deployment in hospitals. Government-led incentives and favorable trade policies are improving access to imported technologies, particularly cartridge-based PCR kits. A key local example includes Brazil’s adoption of AI-powered diagnostic dashboards to track dengue outbreaks in real time. Consumer behavior is shaped by community-driven demand, with urban populations preferring rapid tests for immediate results, while public hospitals lean on molecular platforms for confirmatory diagnostics.
Why is modernization crucial for diagnostic expansion in this market?
Middle East & Africa represented 5.9% of the global market share in 2024, with growth led by the UAE and South Africa. Regional demand is driven by increasing outbreaks in tropical belts and government-backed investments in vector control. Solar-powered PCR devices and mobile diagnostic units are expanding access across underserved regions, covering more than 15 million people. Local regulations have accelerated approvals for emergency-use rapid kits, ensuring timely outbreak response. Certest Biotech SL is partnering with African distribution channels to expand availability of affordable antigen kits. Consumer behavior indicates higher reliance on mobile clinics and NGO-supported field diagnostics, with urban hospitals focusing on molecular confirmation for severe infection cases.
United States – 29.4% Market Share
Strong nationwide vector surveillance programs and advanced molecular diagnostic infrastructure support its leadership.
China – 15.7% Market Share
High manufacturing capacity and rapid deployment of cost-effective diagnostic kits across both urban and rural centers sustain its dominance.
The Mosquito-Borne Infections Testing Market is moderately consolidated, with 8–12 globally active firms competing across North America, Europe, and Asia-Pacific. The top 5 companies collectively account for nearly 54% of the market share, with Abbott Laboratories and Roche leading in molecular diagnostics, while Thermo Fisher Scientific and InBios specialize in rapid and serological formats. Strategic initiatives include emergency supply partnerships with governments, cross-border collaborations to standardize testing, and mergers to expand assay portfolios. Innovation is evident in integrated sample-to-answer platforms, AI-driven result interpretation, and cartridge-based rapid PCR. Competitive intensity is rising as regional players from Asia-Pacific scale low-cost production, putting pricing pressure on global incumbents. As sustainability gains prominence, eco-friendly test kits and digital-first diagnostic systems are shaping future positioning, making agility and scalability key to competitive advantage.
NovaTec Immundiagnostica GmbH
Abnova Corporation
Roche (F. Hoffmann-La Roche Ltd.)
Certest Biotech SL
OriGene Technologies
The technology landscape in mosquito-borne infections testing is rapidly evolving, reshaping diagnostics from centralized labs to field-ready models. Molecular diagnostics remain the backbone, with cartridge-based multiplex PCR capable of detecting up to four arboviruses in a single run, reducing testing time by nearly 50%. Automated nucleic acid extraction-to-PCR workflows have minimized technician involvement by 40%, increasing throughput for reference laboratories. Rapid antigen and serological tests are advancing with AI-enhanced lateral flow readers and smartphone-based interpretation tools, which improve accuracy by 25% and enable digital reporting integration. Portable isothermal amplification systems, designed for rural deployments, can confirm infections within 30 minutes and operate on rechargeable batteries.
Next-generation sequencing is gaining traction in regional hubs, supporting pathogen discovery and variant monitoring across outbreaks. In parallel, sustainability-focused innovations—such as biodegradable plastics in test cassettes and energy-efficient thermocyclers—are becoming mainstream. By 2024, more than 35% of diagnostic manufacturers integrated digital surveillance features into their platforms, allowing real-time data sharing with health authorities. The convergence of AI, portable molecular instruments, and sustainable manufacturing is setting the foundation for a future where diagnostic accessibility, speed, and environmental responsibility converge.
In April 2024, a diagnostics manufacturer introduced a portable isothermal PCR device capable of detecting dengue, Zika, and chikungunya in under 30 minutes with a 98% concordance rate, significantly improving outbreak preparedness. Source: www.medicaldevice-network.com
In December 2024, a global player launched an AI-enabled lateral flow reader that improved accuracy by 25% and automatically logged results to cloud-based surveillance databases for streamlined case reporting. Source: www.diagnosticsworldnews.com
In March 2024, a biotechnology firm entered a public-private partnership with a national ministry of health to roll out multiplex RT-PCR panels across 100 regional labs, enhancing arbovirus surveillance.
In September 2023, a diagnostics company unveiled an eco-friendly rapid serology cassette using biodegradable plastic, reducing medical waste by 30% in community testing programs. Source: www.lab-innovations.com
This Mosquito-Borne Infections Testing Market Report provides a comprehensive view of diagnostic innovation, adoption trends, and regional demand drivers. The scope covers all major test types, including molecular diagnostics, serological assays, rapid antigen kits, ELISA systems, microarrays, and next-generation sequencing. Applications include clinical diagnosis, public health surveillance, outbreak response, and genomic research.
Geographically, the report spans North America, Europe, Asia-Pacific, South America, and Middle East & Africa, with detailed analysis of infrastructure, adoption rates, and consumer behavior. Segment-level insights examine technology share—such as molecular diagnostics accounting for 46% adoption in 2024—as well as emerging areas like portable isothermal amplification and AI-enabled test interpretation. The study also evaluates end-user segments, with public health labs holding 49% share, hospitals contributing 26%, and mobile diagnostic units expanding fastest.
Strategically, the report highlights the role of sustainability, government funding, and digital transformation in shaping market growth. Emerging opportunities include solar-powered field labs, multiplex PCR scalability, and algorithmic serology pathways. By consolidating regional dynamics, technology evolution, and competitive positioning, the report offers actionable intelligence for decision-makers planning investments, partnerships, and regulatory-aligned strategies within this evolving sector.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2024) | USD 2,682.0 Million |
| Market Revenue (2032) | USD 3,606.1 Million |
| CAGR (2025–2032) | 3.77 % |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User
|
| Key Report Deliverables | Revenue Forecast, Growth Drivers & Restraints, Technology Insights, Market Dynamics, Segmentation Analysis, Regional Insights, Competitive Landscape, Recent Developments, Scope of Report |
| Regions Covered | North America; Europe; Asia-Pacific; South America; Middle East & Africa |
| Key Players Analyzed | Abbott Laboratories, Thermo Fisher Scientific, InBios International, NovaTec Immundiagnostica (GmbH), Abnova Corporation, Roche (F. Hoffmann-La Roche Ltd.), Certest Biotech SL, OriGene Technologies |
| Customization & Pricing | Available on Request (10% Customization is Free) |
