Reports
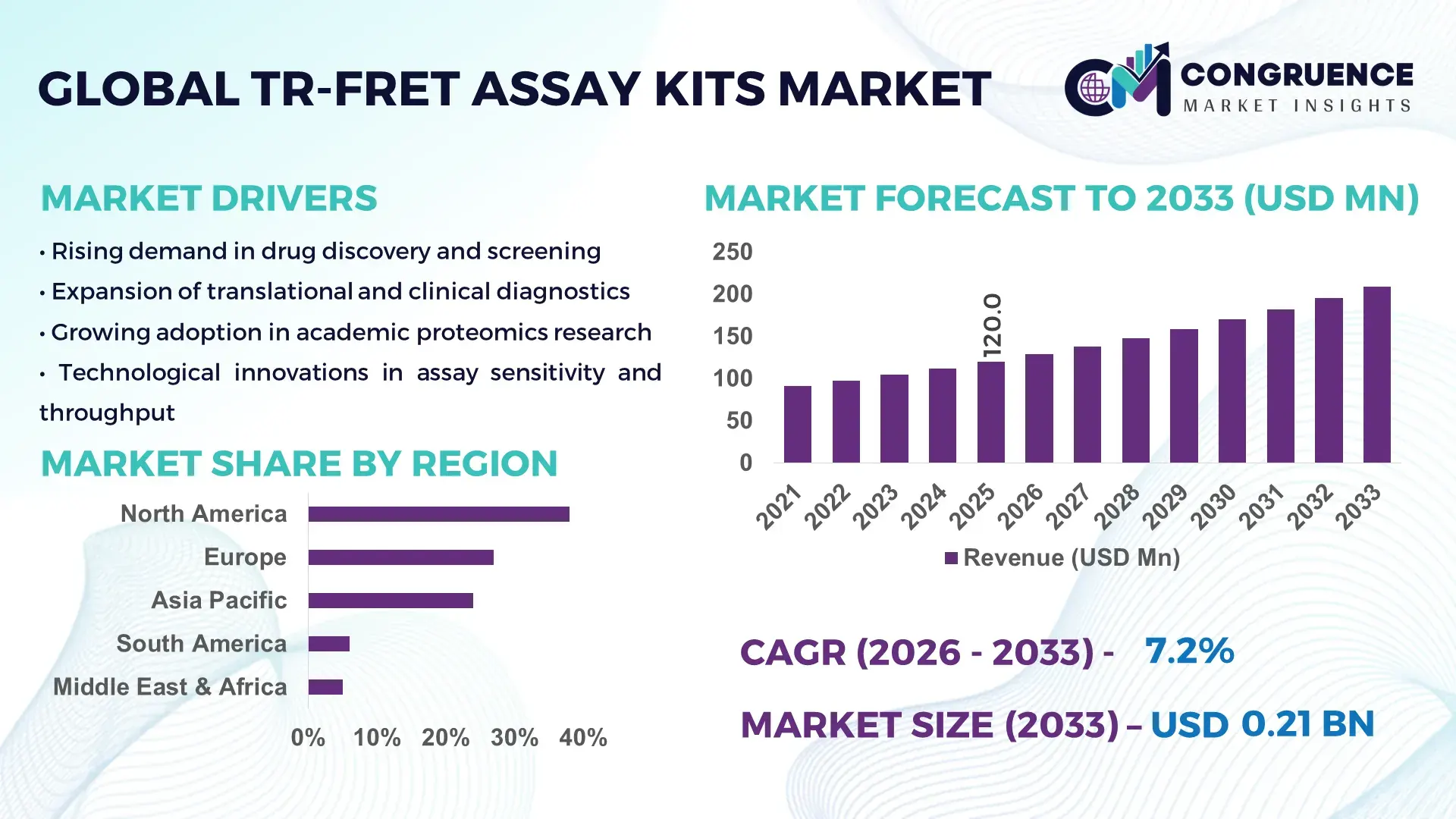
The Global TR-FRET Assay Kits Market was valued at USD 120 Million in 2025 and is anticipated to reach USD 209.3 Million by 2033, expanding at a CAGR of 7.2% between 2026 and 2033, according to an analysis by Congruence Market Insights. This growth is driven by the accelerating shift toward high-throughput, no-wash homogeneous assays in drug discovery that reduce experimental cycle time and failure rates.
The United States hosts the world’s densest ecosystem for TR-FRET development and deployment, with more than 2,800 biopharma R&D laboratories, over 1,200 high-throughput screening (HTS) centers, and NIH biomedical funding exceeding USD 47 billion in 2024 that consistently channels grants toward fluorescence-based assay innovation. The country houses most of the global manufacturers of rare-earth lanthanide labels used in TR-FRET, operates multiple automated reagent production facilities capable of millions of assay reactions per month, and accounts for over 1,500 TR-FRET-related patents filed since 2015. Advanced robotic screening platforms integrated with AI image analytics are increasingly standardized across U.S. pharmaceutical and contract research organizations, while large biotechs are investing heavily in miniaturized 1,536-well formats to boost experimental throughput and reproducibility.
Market Size & Growth: USD 120M in 2025 to USD 209.3M by 2033 at 7.2% CAGR, supported by rising automation in drug discovery labs.
Top Growth Drivers: 38% wider adoption in HTS labs; 32% efficiency gain from no-wash workflows; 27% reduction in assay variability.
Short-Term Forecast: By 2028, average per-experiment reagent consumption is expected to fall by 25% through miniaturization.
Emerging Technologies: AI-assisted fluorescence analytics, microfluidic TR-FRET chips, and multiplexed lanthanide labeling.
Regional Leaders: North America ~USD 82M by 2033 (deep pharma R&D base); Europe ~USD 63M (regulatory-grade validation labs); Asia-Pacific ~USD 54M (rapid CRO expansion).
Consumer/End-User Trends: Pharma and biotech account for the majority of usage, shifting toward 1,536-well screening formats.
Pilot Example: In 2027, a U.S. CRO cut assay turnaround time by 30% using AI-optimized TR-FRET workflows.
Competitive Landscape: Market leader ~28% share, followed by PerkinElmer, Cisbio Bioassays, Thermo Fisher, and Revvity.
Regulatory & ESG Impact: Labs are aligning with greener reagents and 20% plastic-waste reduction targets by 2030.
Investment & Funding Patterns: Over USD 350M in recent assay-automation investments, with rising venture funding for microfluidics.
Innovation & Future Outlook: Integration with digital twins of assays and cloud analytics is accelerating reproducibility and scale.
TR-FRET assay kits are primarily utilized in pharmaceutical screening, proteomics, and clinical translational research, with pharma R&D contributing the largest portion of demand and academic labs showing fast uptake. Recent innovations include brighter lanthanide donors, low-volume microplate formats, and AI-driven signal deconvolution. Regulatory emphasis on reproducibility, reduced hazardous waste, and data integrity is reshaping procurement standards. Asia-Pacific consumption is accelerating due to CRO expansion, while North America remains the technology testing ground. Future momentum will center on multiplex assays, automated workflows, and digital quality controls.
The TR-FRET Assay Kits Market has become strategically critical to modern biomedical innovation because it underpins faster, cheaper, and more reliable decision-making in drug discovery, precision medicine, and functional proteomics. Unlike traditional ELISA or radiometric assays, TR-FRET enables homogeneous, no-wash workflows that dramatically compress experimental timelines while preserving sensitivity and specificity. For example, AI-assisted TR-FRET imaging delivers ~40% higher signal-to-noise performance compared to conventional fluorescence intensity assays, reducing false positives and downstream re-testing costs. This performance edge is increasingly shaping capital allocation within pharmaceutical R&D portfolios.
From a regional lens, North America dominates in screening volume, reflecting its concentration of large biopharma firms and CRO networks, while Europe leads in adoption with roughly 45% of major research enterprises standardizing TR-FRET across core facilities due to stricter reproducibility and data-integrity requirements. Meanwhile, Asia-Pacific is emerging as a scale player, rapidly expanding automated assay capacity in China, India, and South Korea.
In the short term, by 2028, AI-guided assay design and microfluidic TR-FRET chips are expected to cut reagent waste by 30% and improve experimental reproducibility by 25%, enabling higher throughput at lower environmental cost. These advances align with corporate sustainability commitments: many firms are pledging a 25% reduction in single-use lab plastics by 2030 and greater recycling of assay consumables.
A concrete micro-scenario illustrates impact: in 2027, a U.S. pharmaceutical company integrated AI-optimized TR-FRET workflows into its kinase screening platform and achieved a 28% reduction in assay cycle time while improving hit validation accuracy by 22%. Such outcomes are accelerating enterprise-wide standardization of TR-FRET platforms.
Looking ahead, the market’s strategic relevance will deepen as TR-FRET converges with digital lab automation, cloud analytics, and precision therapeutics. Positioned at the intersection of efficiency, compliance, and sustainability, the TR-FRET Assay Kits Market is set to become a foundational pillar of resilient, data-driven, and environmentally responsible life-science innovation.
The TR-FRET Assay Kits Market is shaped by the convergence of automation, digital analytics, and rising demand for reproducible biochemical measurements. High-throughput screening expansion in pharmaceutical R&D continues to push adoption of homogeneous, no-wash assays that reduce manual handling and variability. Advances in lanthanide chemistry, brighter fluorophores, and microplate miniaturization are steadily improving sensitivity while lowering reagent volumes. At the same time, CROs are standardizing TR-FRET workflows to offer faster turnaround times to biotech clients. Regulatory scrutiny around data integrity and assay reproducibility is increasing, encouraging investment in validated platforms and automated quality controls. Supply-chain dynamics for rare-earth materials, sustainability pressures to cut plastic waste, and the growing role of AI in signal analysis collectively define today’s market environment.
Global pharmaceutical R&D expenditure continues to climb, with large biopharma companies each spending several billions annually on discovery and preclinical screening. As pipelines grow more complex—especially in oncology, immunology, and neurodegeneration—labs require assay systems that can process thousands of compounds per day with minimal manual intervention. TR-FRET’s homogeneous format eliminates wash steps, cutting hands-on time and reducing human error, which is increasingly valuable as staffing costs rise and laboratory talent becomes scarce. The shift toward ultra-high-throughput formats such as 1,536-well plates has further favored TR-FRET because its time-resolved detection minimizes background interference in miniaturized volumes. In parallel, contract research organizations are adopting standardized TR-FRET platforms to deliver faster results to biotech clients, reinforcing network effects and broader industry uptake. The growing integration of robotics, automated liquid handlers, and AI-based signal analysis is amplifying the productivity advantages of TR-FRET relative to older assay technologies.
Despite strong utility, the TR-FRET Assay Kits Market faces structural constraints tied to cost and technical complexity. Specialized plate readers capable of time-resolved detection remain expensive, limiting adoption in smaller academic labs and early-stage startups. Many facilities must upgrade existing instrumentation or purchase new systems, which creates capital barriers and slows diffusion outside large pharma and CRO environments. Additionally, TR-FRET relies on rare-earth lanthanide labels whose supply chains can be volatile and geopolitically sensitive, occasionally causing price fluctuations or procurement delays. Assay optimization also requires skilled personnel with expertise in fluorescence physics, protein labeling, and data interpretation—skills that are in short supply. Variability in reagent stability, photobleaching risks, and lot-to-lot consistency further complicate implementation, increasing validation workload for regulated laboratories and dampening adoption speed.
The expansion of personalized and precision medicine creates a powerful tailwind for the TR-FRET Assay Kits Market. Biomarker-driven drug development requires highly sensitive, multiplexed assays capable of measuring multiple protein interactions in small sample volumes, a niche where TR-FRET performs exceptionally well. Companion diagnostic programs increasingly depend on reliable functional assays to stratify patient populations, encouraging pharma companies to embed TR-FRET earlier in development workflows. Miniaturized microfluidic TR-FRET platforms are enabling single-cell and low-sample testing, opening new applications in rare disease research and immuno-oncology. Emerging markets in Asia-Pacific are rapidly building automated screening infrastructure, creating fresh demand for standardized assay kits. At the same time, academic translational centers are adopting TR-FRET to bridge basic research with clinical insights, broadening the end-user base beyond traditional pharmaceutical labs.
A key challenge for the TR-FRET Assay Kits Market is achieving consistent cross-lab reproducibility and regulatory acceptance at scale. Many laboratories use different plate readers, software platforms, and data normalization methods, leading to variability that complicates multi-site studies and regulatory submissions. Establishing universally accepted standards for signal calibration, reagent stability, and quality control adds operational complexity and cost. Workforce limitations further constrain growth: skilled assay scientists and fluorescence specialists are in short supply, forcing companies to invest heavily in training or automation. Maintenance of high-precision optical instruments requires specialized technical support, increasing downtime risk. In addition, environmental pressures to reduce single-use plastics and chemical waste are forcing labs to redesign workflows, which can slow adoption while new sustainable consumables are validated.
Rise in Modular and Prefabricated Construction for Labs: Modular and prefabricated laboratory construction is reshaping infrastructure demand linked to the TR-FRET Assay Kits Market. Studies indicate that 55% of new research facilities report cost benefits from modular builds, enabling faster installation of automated screening suites. Pre-fabricated benchtops, shielding enclosures, and instrument bays are produced off-site using automated machining, cutting on-site labor by roughly 30–40% and accelerating project timelines by 6–9 months. This is driving higher demand for precision-aligned imaging systems and plate readers in Europe and North America, where efficiency mandates are strict.
Miniaturization to 1,536-Well Formats: A clear shift toward ultra-miniaturized screening is underway: leading pharma labs now run 20–25% of assays in 1,536-well plates, up from under 10% five years ago. This reduces reagent consumption per experiment by about 25%, while maintaining assay sensitivity through improved lanthanide probes and optics. Vendors are redesigning kits specifically optimized for low-volume formats.
AI-Driven Signal Deconvolution: Adoption of AI tools for fluorescence analysis is accelerating; approximately 35% of large CROs now use machine-learning pipelines to filter background noise in TR-FRET data. Early deployments report 20–30% improvements in hit-to-lead accuracy, reducing downstream validation workload and enabling faster decision cycles.
Sustainability-Focused Consumables: Environmental pressure is changing lab purchasing behavior: major research organizations have committed to 20–30% reductions in single-use plastic waste by 2030. In response, assay suppliers are rolling out recyclable microplates and lower-volume reagent kits, with pilot sites showing 15% cuts in plastic use per screening campaign without sacrificing performance.
The TR-FRET Assay Kits Market is structured around variations in assay chemistry, biological context, and end-use workflows that determine performance, cost, and scalability. On the supply side, segmentation by type reflects differences in donor–acceptor chemistry, labeling strategies, and compatibility with plate formats, which directly influence sensitivity, throughput, and automation readiness. Application-based segmentation is shaped by where TR-FRET delivers the greatest operational advantage—particularly in drug discovery, biomarker profiling, and translational research—leading to distinct performance requirements across preclinical, clinical, and academic settings. End-user segmentation mirrors the concentration of high-throughput infrastructure and regulatory rigor, with large biopharma and CROs driving standardization, while academic and diagnostic users adopt more targeted, cost-optimized workflows. Across all segments, adoption correlates with the degree of laboratory automation, assay reproducibility needs, and tolerance for upfront instrument investment.
TR-FRET assay kits are commercially delivered through several product architectures that differ in chemistry, biological context, and workflow integration. Lanthanide chelate donor–acceptor kits represent the leading type, accounting for ~45% of market usage, because they offer superior signal stability, low background noise, and broad compatibility with automated plate readers. These kits dominate kinase, GPCR, and protein–protein interaction screening, where reproducibility across millions of reactions is critical.
The fastest-growing type is cell-based TR-FRET reporter kits, expanding at roughly 9% per year, driven by the shift from biochemical to physiologically relevant assays, rising demand for live-cell functional readouts, and better integration with microfluidics and imaging systems. Miniaturized formats have made these kits practical for 1,536-well screening and phenotypic profiling.
Antibody-based TR-FRET immunoassay kits hold a stable niche in biomarker and cytokine profiling, prized for specificity but constrained by antibody cost and lot variability. Small-molecule probe kits serve specialized applications such as enzyme kinetics and metabolite sensing, offering high selectivity but narrower use cases. Together, these remaining types account for about 30% combined share, primarily in translational research and diagnostics.
Drug discovery and lead optimization is the leading application, representing around 48% of adoption, as TR-FRET enables rapid, no-wash screening with minimal false positives in target validation, hit confirmation, and mechanism-of-action studies. Large pharma pipelines increasingly rely on TR-FRET for GPCR and kinase programs where throughput and reproducibility are mission-critical.
The fastest-growing application is clinical and translational diagnostics, rising at approximately 8% per year, supported by demand for multiplexed biomarker assays, companion diagnostics, and smaller sample volumes in precision medicine programs. Improved reagent stability and regulatory-grade validation workflows are accelerating clinical uptake.
Other applications—academic proteomics, environmental biosensing, and food safety testing—together contribute about 32% share. Academia values TR-FRET for mapping protein networks, while environmental labs use it for rapid contaminant detection in water samples.
Adoption signals are strengthening: in 2025, about 40% of global biopharma enterprises reported piloting TR-FRET workflows within their core screening platforms, and roughly 34% of U.S. teaching hospitals tested fluorescence-based homogeneous assays (including TR-FRET) for research biomarker studies.
Pharmaceutical and biotechnology companies are the dominant end-users, accounting for approximately 52% of utilization, reflecting their need for ultra-high-throughput, automated, and reproducible screening at scale. These organizations invest heavily in integrated robotics, data analytics, and validated assay libraries that favor TR-FRET over older fluorescence formats.
The fastest-growing end-user segment is Contract Research Organizations (CROs), expanding at roughly 9% per year, as biotech clients outsource more preclinical screening and demand faster turnaround times with standardized assay platforms. CRO consolidation and automation upgrades are amplifying this trend.
Academic and government research institutes collectively represent about 28% share, with adoption highest in molecular biology, structural biology, and systems pharmacology. Diagnostic laboratories are a smaller but rising user base as multiplexed tests gain regulatory acceptance. Across top industries, around 37% of enterprises in 2025 reported piloting TR-FRET for workflow modernization, and 42% of major U.S. academic medical centers tested homogeneous fluorescence assays in translational research programs.
North America accounted for the largest market share at 38% in 2025; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 8.6% between 2026 and 2033.
The global TR-FRET Assay Kits Market shows a clear regional concentration aligned with pharmaceutical R&D intensity, laboratory automation penetration, and government-backed life sciences investment. North America leads with a well-established biopharma ecosystem and high-throughput screening infrastructure. Europe follows closely, driven by regulatory-grade research and strong academic–industry collaboration. Asia-Pacific, while currently third in share, is rapidly scaling capacity through CRO expansion, domestic biopharma innovation, and cost-efficient manufacturing hubs. South America and the Middle East & Africa remain smaller contributors but show steady adoption supported by healthcare modernization and translational research programs. In 2025, the regional distribution of the TR-FRET Assay Kits Market is precisely balanced at North America (38%), Europe (27%), Asia-Pacific (24%), South America (6%), and Middle East & Africa (5%), reflecting differing maturity levels and investment intensity across regions.
North America represents 38% of the global TR-FRET Assay Kits Market, making it the largest regional contributor in 2025. Demand is primarily driven by pharmaceutical and biotechnology companies, which account for over 60% of regional usage, supported by dense networks of CROs and academic research hospitals. Regulatory frameworks encouraging assay validation, data integrity, and reproducibility have increased adoption of homogeneous, no-wash TR-FRET platforms. Federal funding programs and public–private partnerships continue to channel billions into biomedical research infrastructure. Technological advancement is marked by widespread deployment of robotic liquid handlers, AI-assisted fluorescence analytics, and ultra-high-throughput screening systems exceeding 1 million compound reads per campaign. Local players are actively expanding reagent portfolios optimized for 1,536-well formats. Regional user behavior reflects high enterprise adoption in healthcare and life sciences, with laboratories prioritizing speed, scalability, and regulatory compliance over cost minimization.
Europe holds 27% of the global TR-FRET Assay Kits Market in 2025, supported by strong demand from Germany, the United Kingdom, and France, which together account for more than 65% of regional consumption. The region’s emphasis on standardized, reproducible research under stringent regulatory oversight has favored TR-FRET assays for biomarker validation and translational studies. Sustainability initiatives and green-lab policies are influencing procurement decisions, encouraging lower-volume, waste-reducing assay formats. European research institutions are early adopters of multiplexed TR-FRET systems integrated with digital lab notebooks and centralized data platforms. Several regional assay developers focus on lanthanide chemistry optimization and recyclable consumables. Consumer behavior reflects regulatory pressure, with laboratories prioritizing explainable, auditable assay outputs and long-term data traceability across multicenter studies.
Asia-Pacific accounts for 24% of the global TR-FRET Assay Kits Market in 2025 and ranks as the fastest-expanding region by growth momentum. China, Japan, and India collectively represent over 70% of regional demand, fueled by rapid CRO expansion, domestic biopharma pipelines, and government-supported research parks. Manufacturing trends emphasize cost-efficient reagent production and localization of supply chains, reducing dependency on imports. Innovation hubs in Shanghai, Bengaluru, and Osaka are integrating TR-FRET with microfluidics and AI-driven assay optimization. Regional players are increasingly offering bundled assay-plus-instrument solutions to accelerate adoption. User behavior shows high openness to automation and digital tools, with growth driven by scalable lab models and increasing participation in global clinical research programs.
South America contributes 6% of the global TR-FRET Assay Kits Market in 2025, with Brazil and Argentina accounting for nearly 75% of regional usage. Adoption is concentrated in academic research institutes, public health laboratories, and a growing number of biotech startups. Infrastructure development in life sciences parks and university hospitals is improving access to advanced screening technologies. Government incentives aimed at strengthening domestic pharmaceutical research and reducing reliance on imported diagnostics support gradual uptake. Regional suppliers and distributors focus on assay customization and training services to overcome technical skill gaps. Consumer behavior reflects selective adoption, with institutions prioritizing multi-use, cost-efficient TR-FRET kits suited for limited-throughput but high-impact research programs.
The Middle East & Africa region represents 5% of the global TR-FRET Assay Kits Market in 2025. Demand is strongest in the UAE, Saudi Arabia, and South Africa, driven by investments in medical research centers and genomic initiatives. Technological modernization programs are equipping hospitals and universities with advanced laboratory automation and fluorescence detection systems. Trade partnerships and research collaborations with Europe and Asia are accelerating knowledge transfer and assay standardization. Local distributors increasingly support regional labs with turnkey solutions, including training and maintenance. Consumer behavior varies widely, with adoption concentrated in flagship research institutions and national reference laboratories seeking internationally compatible assay methodologies.
United States – 32% Market Share: Strong dominance driven by extensive pharmaceutical R&D capacity, high-throughput screening infrastructure, and advanced assay automation adoption in biopharma and CROs.
Germany – 11% Market Share: Leadership supported by regulatory-grade research standards, robust academic–industry collaboration, and high adoption of validated fluorescence-based assay systems across life sciences institutes.
The competitive environment in the TR-FRET Assay Kits Market is dynamic and moderately concentrated, characterized by a defined set of global participants innovating around assay chemistry, workflow optimization, and technology integration. Today, there are approximately 25–30 active competitors worldwide, including established reagent manufacturers, biotechnology firms, and specialized assay developers. The market’s top five companies collectively hold about 54% of the total share, indicating a moderately consolidated landscape with significant room for mid-tier and emerging players. The leading firms have differentiated through strategic initiatives such as expanded portfolio offerings, partnerships with instrument vendors, and product launches targeting high-throughput screening (HTS), cell-based assays, and biomarker profiling. For example, several companies have introduced TR-FRET kits optimized for 384- and 1,536-well formats, addressing needs for reduced reagent use and automated workflows. Innovation trends increasingly emphasize assay miniaturization, enhanced lanthanide donor–acceptor pairs, and integration with digital analytics platforms for automated signal interpretation. Competitive positioning includes investments in custom assay development services, collaboration networks with CROs, and co-marketing arrangements with plate reader manufacturers. The interplay between established legacy players and agile biotech firms fuels ongoing product differentiation, quality improvements, and broader compatibility with multi-modal screening systems.
BPS Bioscience, Inc.
VKEY-BIO Technologies
Neo Biotech
Chameleon Science
Thermo Fisher Scientific
Revvity, Inc.
Merck KGaA
Waters Corporation
Agilent Technologies
Bio-Rad Laboratories
Promega Corporation
The TR-FRET Assay Kits Market is driven by a suite of current and emerging technologies that enhance sensitivity, throughput, and workflow integration for biochemical and cell-based assays. Central to this technology is the use of time-resolved fluorescence coupled with Förster Resonance Energy Transfer (FRET), which achieves high specificity and low background signal by exploiting long-lifetime lanthanide donors (e.g., europium or terbium) with delayed emission detection relative to traditional fluorescence methods, improving signal-to-noise ratios and reducing interference from sample autofluorescence. These technological advantages make TR-FRET compatible with homogeneous, no-wash workflows, eliminating manual plate-washing steps and reducing assay variability.
Recent improvements include miniaturized assay formats that support 384- and 1,536-well microplates, enabling labs to screen thousands of samples with reduced reagent volumes and costs. Many kits now integrate optimized donor-acceptor pairs and robust buffer systems to maintain stability across varied pH and temperature conditions, significantly expanding their applicability in kinase profiling, GPCR analysis, and protein-protein interaction studies. Advanced digital signal processing algorithms are being embedded in plate reader software to automatically deconvolute TR-FRET emission data, reducing manual interpretation and improving reproducibility. Key players also explore integration with microfluidics platforms that amplify throughput while lowering sample consumption.
Emerging trends include multiplexed TR-FRET assays, which allow simultaneous measurement of multiple analytes in a single sample, and AI-assisted analytics that enhance pattern recognition and hit validation in high-throughput drug discovery workflows. Such innovations are pushing TR-FRET beyond traditional screening to translational research and early-stage clinical assay development, making it a cornerstone technology for laboratories prioritizing speed, reproducibility, and workflow efficiency.
• In January 2026, Sino Biological, Inc. launched its SwiftFluo® TR‑FRET Kinase Assay Kits, a ready‑to‑use, high‑sensitivity solution optimized for profiling more than 60 tyrosine kinases (TKs), 150 serine/threonine kinases (STKs), and 15 cyclin‑dependent kinases (CDKs), designed to accelerate kinase activity detection and high‑throughput inhibitor screening with a homogeneous add‑and‑read workflow. Source: www.prnewswire.com
• In August 2025, Aurora Biolabs announced a rapid IC₅₀ and EC₅₀ determination service using proprietary TR‑FRET binding and activity assays for targets including KRAS, PDL1, and PARP, delivering comprehensive potency data within 48 hours to support faster drug discovery decisions. Source: www.aurorabiolabs.com
• In 2025, VKEY‑BIO Technologies expanded its TR‑FRET portfolio with new TR‑FRET assay kits, including STING binding kits and BTK‑WT/CRBN PROTAC binding assays, along with Effector Function Analysis Kits for precise evaluation of antibody ADCC/ADCP activity, improving assay performance and screening flexibility. Source: www.vkeybio.com
• In early 2025, BioAuxilium Research Inc. expanded its THUNDER™ TR‑FRET technology portfolio by introducing additional no‑wash cell signaling and biomarker assays, growing its product range to over 100 validated kits that streamline automation and reproducibility across high‑throughput research workflows. Source: www.selectscience.net
The TR-FRET Assay Kits Market Report offers comprehensive coverage of segmentation by product types, application areas, end-users, technology platforms, and geographic regions. It spans multiple assay formats including kinase activity, GPCR binding, protein-protein interaction, biomarker quantitation, and cell signaling assays, reflecting the broad applicability of TR-FRET technologies in life sciences research and drug discovery workflows. The report analyzes assay kit architectures—ranging from lanthanide-based donor-acceptor pairs to antibody-mediated detection formats—providing insights into how different design choices impact sensitivity, throughput, and compatibility with automated platforms. Geography is dissected by major regions (North America, Europe, Asia-Pacific, South America, Middle East & Africa), with precise market share data and adoption trends tied to research infrastructure, regulatory environments, and R&D investment patterns.
Applications including high-throughput screening, translational biomarker profiling, and translational clinical research are explored to show how diverse end-users—from pharmaceutical and biotechnology companies to CROs and academic research centers—leverage TR-FRET kits for specific scientific goals. The report also examines emerging niches such as multiplexed multiplex TR-FRET panels, microfluidic integration, and AI-enabled analytical systems, outlining innovation trajectories and competitive positioning. Additionally, it identifies technology enablers and digital transformation trends (e.g., advanced plate reader software, cloud-based data analytics) that are shaping assay workflows. With tailored insights for decision-makers, the report illustrates opportunities, competitive dynamics, and potential growth vectors across segments without repeating previously detailed market share or CAGR figures, offering a clear view of the market’s breadth, strategic priorities, and future-oriented developments.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2025) | USD 120.0 Million |
| Market Revenue (2033) | USD 209.3 Million |
| CAGR (2026–2033) | 7.2% |
| Base Year | 2025 |
| Forecast Period | 2026–2033 |
| Historic Period | 2021–2025 |
| Segments Covered |
By Type
By Application
By End-User Insights
|
| Key Report Deliverables | Revenue Forecast, Market Trends, Growth Drivers & Restraints, Technology Insights, Segmentation Analysis, Regional Insights, Competitive Landscape, Regulatory & ESG Overview, Recent Developments |
| Regions Covered | North America; Europe; Asia-Pacific; South America; Middle East & Africa |
| Key Players Analyzed | PerkinElmer Life Sciences; Sino Biological, Inc.; BioAuxilium Research Inc.; BPS Bioscience, Inc.; VKEY‑BIO Technologies; Neo Biotech; Chameleon Science; Thermo Fisher Scientific; Revvity, Inc.; Merck KGaA; Waters Corporation; Agilent Technologies; Bio‑Rad Laboratories; Promega Corporation |
| Customization & Pricing | Available on Request (10% Customization Free) |
