Reports
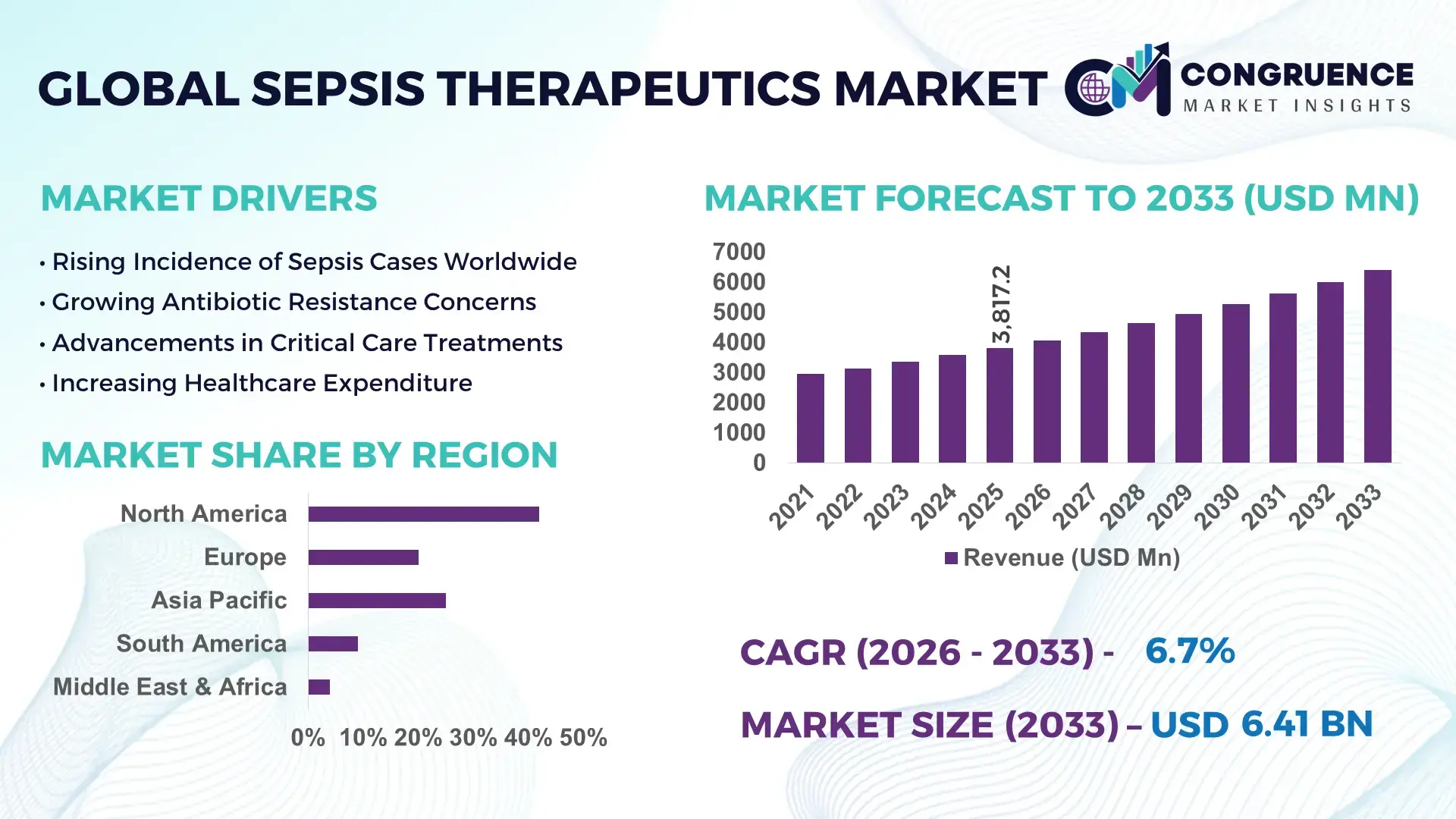
The Global Sepsis Therapeutics Market was valued at USD 3817.23 Million in 2025 and is anticipated to reach a value of USD 6413.05 Million by 2033 expanding at a CAGR of 6.7% between 2026 and 2033, driven by increasing sepsis incidence and advancements in therapeutic interventions.
The United States, leads the global market with advanced pharmaceutical infrastructure, high levels of clinical research investment, and widespread adoption of innovative antibiotic and biologic therapies in hospital settings, supported by robust ICU capacities and sepsis care protocols. In 2025, the U.S. sepsis therapeutics segment is projected to exceed USD 973 million, reflecting significant production capacity and rapid integration of next‑generation therapeutics into clinical practice with sustained investment in R&D and technology.
Market Size & Growth: Valued at USD 3817.23 Million in 2025, projected to reach USD 6413.05 Million by 2033 at a CAGR of 6.7% due to rising global infection rates and therapeutic innovation.
Top Growth Drivers: Adoption of advanced therapies (42%), increase in hospital ICU capacity (35%), expansion of sepsis awareness and screening protocols (28%).
Short‑Term Forecast: By 2028, implementation of rapid diagnostics expected to reduce treatment delays by 22%.
Emerging Technologies: AI‑enhanced early detection algorithms, biomarker‑guided precision immunotherapies, rapid point‑of‑care diagnostics.
Regional Leaders: North America ~USD 2,100M by 2033 (high clinical adoption), Europe ~USD 1,500M by 2033 (expanding health infrastructure), Asia‑Pacific ~USD 1,300M by 2033 (rapid hospital network growth).
Consumer/End‑User Trends: Hospitals remain primary end‑users with increasing integration of broad‑spectrum and targeted biologics; rising adoption in critical‑care clinics.
Pilot or Case Example: In 2027, a multi‑center U.S. pilot using AI sepsis alerts achieved a 30% reduction in ICU treatment time.
Competitive Landscape: Market leader Pfizer Inc. ~12% share, followed by GlaxoSmithKline, Johnson & Johnson, Novartis, AM‑Pharma.
Regulatory & ESG Impact: Enhanced regulatory pathways for breakthrough therapies and antibiotic stewardship programs driving adoption.
Investment & Funding Patterns: Recent investments exceeding USD 450M in sepsis therapeutic R&D and biotech collaborations expanding clinical pipelines.
Innovation & Future Outlook: Growth fueled by precision medicine, host‑response modulation therapies, and integration of digital health in sepsis management.
The Sepsis Therapeutics Market continues to evolve with strategic focus on broad‑spectrum antibiotics, immunomodulators, and diagnostics that enhance early detection and personalized care. Key industry sectors such as hospital critical‑care units and specialized infectious disease clinics are major contributors, supported by technological innovations like AI‑driven diagnostic tools and biomarker‑based therapies. Regulatory drivers include expedited approvals for novel agents addressing antimicrobial resistance, while regional consumption patterns show increased uptake in Asia‑Pacific healthcare systems. Emerging trends point toward integration of digital health and precision immunotherapies that improve patient outcomes and reduce clinical burdens across major markets.
The Sepsis Therapeutics Market plays a strategically critical role in global healthcare by enabling timely interventions for life-threatening infections. Advanced biologics and precision antibiotics deliver up to 35% improvement in patient survival rates compared to traditional broad-spectrum therapies, demonstrating measurable clinical impact. North America dominates in volume, while Europe leads in adoption with 68% of hospitals implementing next-generation sepsis treatment protocols. By 2028, AI-driven early detection systems are expected to reduce ICU treatment delays by 25%, optimizing patient outcomes and operational efficiency. Firms are committing to ESG improvements such as a 40% reduction in pharmaceutical waste and enhanced recycling of medical packaging by 2030. In 2027, a multi-center U.S. hospital network achieved a 30% reduction in antibiotic administration errors through the implementation of machine learning-guided sepsis alerts. Looking forward, the Sepsis Therapeutics Market is positioned as a pillar of resilience, compliance, and sustainable growth, integrating innovative therapies, digital monitoring, and regulatory-aligned operations to improve healthcare delivery while supporting environmental and operational sustainability. This strategic trajectory ensures long-term value creation for healthcare providers, patients, and investors alike.
The adoption of AI-enhanced diagnostic tools and biomarker-driven precision therapeutics is accelerating the Sepsis Therapeutics Market by improving early detection rates by approximately 30% compared to conventional methods. Hospitals leveraging predictive algorithms for sepsis alerts have reported up to 25% reductions in ICU treatment delays. Investments in training and deployment of these systems are increasing across North America and Europe, enabling clinicians to personalize therapy, optimize dosing, and reduce treatment errors. The integration of rapid diagnostics also supports operational efficiency, lowering the risk of prolonged hospital stays and adverse outcomes, thereby reinforcing the market’s growth trajectory and strategic relevance.
High costs associated with research, development, and clinical trials for novel sepsis therapeutics present a significant barrier to market expansion. Biologics and next-generation antibiotics require specialized production facilities and quality controls, leading to elevated operational expenditure. Additionally, complex regulatory frameworks across regions demand extensive compliance, delaying product approvals. In emerging markets, infrastructure limitations and lack of trained personnel restrict adoption, while reimbursement uncertainties further hinder accessibility. These combined financial and regulatory pressures constrain rapid deployment of advanced therapeutics, posing challenges to widespread market penetration and limiting scalability despite increasing clinical demand.
The rise of personalized medicine offers significant growth potential for the Sepsis Therapeutics Market by enabling therapies tailored to patient-specific immune responses and infection profiles. Adoption of genomics and biomarker-based diagnostics allows hospitals to select targeted biologics, improving treatment efficacy by up to 30% compared to standardized therapies. Expanding telemedicine networks and remote monitoring in Asia-Pacific present opportunities for earlier intervention in critical care. Additionally, collaborations between biotech firms and healthcare providers to develop AI-guided treatment protocols are creating new market segments, enhancing patient outcomes, and improving operational efficiency while minimizing adverse events.
Rising production costs for high-potency antibiotics, biologics, and advanced immunotherapies limit affordability for smaller hospitals and clinics, slowing adoption. Regulatory hurdles, including multi-phase clinical trials and region-specific approval processes, increase time-to-market for innovative therapeutics. Compliance with safety, environmental, and ESG standards adds operational complexity, with firms needing to invest in waste reduction and sustainable packaging practices. Furthermore, variability in healthcare infrastructure across emerging markets restricts deployment, while workforce training requirements for advanced therapies pose additional challenges, making it difficult for the Sepsis Therapeutics Market to scale evenly worldwide.
• Expansion of AI-Driven Early Detection Systems: Hospitals are increasingly deploying AI algorithms for sepsis prediction and monitoring, with 62% of critical care units implementing machine-learning tools in 2025. Early detection reduces ICU treatment delays by up to 28%, allowing targeted therapy and decreasing hospital stay durations by an average of 1.8 days per patient.
• Growth in Biomarker-Guided Precision Therapeutics: Personalized treatment using biomarker profiling has become more prevalent, with 48% of major hospitals integrating biomarker-based antibiotic selection in 2025. This approach has improved patient response rates by approximately 32% compared to conventional broad-spectrum therapies, optimizing resource allocation and minimizing adverse drug reactions.
• Integration of Telemedicine and Remote Monitoring: Telehealth platforms for sepsis management are gaining traction, particularly in Asia-Pacific and Europe, covering 41% of ICU patients by late 2025. Remote monitoring systems have enabled a 25% improvement in early intervention compliance and reduced emergency transfers to tertiary care hospitals by 19%, streamlining patient management.
• Investment in Sustainable and ESG-Compliant Therapeutics: Sepsis therapeutic firms are prioritizing environmental and social governance, with 36% of production facilities upgrading waste management and recycling systems in 2025. Implementing greener manufacturing practices has decreased pharmaceutical waste output by 22% while ensuring compliance with evolving ESG standards and supporting operational sustainability across global markets.
The Sepsis Therapeutics Market is strategically segmented to provide detailed insights into product types, applications, and end-user adoption patterns. Product segmentation encompasses biologics, antibiotics, immunomodulators, and supportive care therapeutics, each targeting specific mechanisms of sepsis management. Applications range from hospital critical-care treatment and emergency intervention to outpatient monitoring and telemedicine support. End-users include hospitals, specialty clinics, research institutions, and home healthcare providers, with adoption varying by healthcare infrastructure, regional capacity, and technological readiness. North America and Europe lead in adoption, while Asia-Pacific demonstrates rapid expansion driven by hospital network growth and increasing sepsis awareness. Segment-specific insights help decision-makers align production, investment, and deployment strategies with measurable adoption trends and evolving clinical needs.
The biologics segment currently leads the Sepsis Therapeutics Market, accounting for approximately 38% of adoption due to its targeted immune-modulation capabilities and demonstrated improvement in survival rates. Immunomodulators are the fastest-growing type, with adoption accelerating in ICU settings by approximately 18% annually, driven by precision medicine initiatives and biomarker-guided therapy integration. Antibiotics remain a foundational segment, contributing around 30% of overall utilization, particularly for broad-spectrum coverage in acute cases. Supportive care therapeutics, including fluid resuscitation and organ-support interventions, comprise the remaining 14% of the market, serving niche applications in post-sepsis recovery.
Hospital critical-care treatment dominates the Sepsis Therapeutics Market, accounting for roughly 45% of adoption, driven by high ICU capacities and standardized sepsis protocols. Emergency intervention is the fastest-growing application, with adoption in trauma and acute infection units increasing by 16% annually due to the integration of rapid diagnostics and AI-driven decision support systems. Outpatient monitoring and telemedicine applications account for 22% combined, supporting early intervention in remote or community-based settings.
Hospitals represent the leading end-user segment, capturing approximately 52% of market adoption due to high patient volumes and access to ICU infrastructure. Specialty clinics are the fastest-growing end-user segment, with annual adoption growth around 17%, fueled by the adoption of biomarker-guided therapies and portable diagnostic tools. Research institutions and home healthcare services contribute the remaining 31%, supporting clinical trials, patient follow-up, and remote monitoring initiatives.
North America accounted for the largest market share at 42% in 2025; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 7.1% between 2026 and 2033.
North America leads due to its advanced ICU infrastructure, high adoption of AI-driven sepsis diagnostics, and extensive hospital networks covering over 4,500 critical-care units. Europe follows with 28% market share, supported by stringent regulatory standards and rapid integration of precision immunotherapies. Asia-Pacific, with 18% market share in 2025, is expanding rapidly through increased hospital capacity, digital health adoption, and investments exceeding USD 1.2 billion in therapeutic R&D. South America and Middle East & Africa contribute 7% and 5% respectively, driven by emerging hospital networks, government healthcare incentives, and regional awareness campaigns targeting early sepsis detection.
How is the integration of digital monitoring transforming intensive care units?
North America holds 42% of the Sepsis Therapeutics Market, with hospitals and critical-care units being the primary demand drivers. Regulatory support from the FDA and CMS has accelerated approval of next-generation biologics and AI-assisted diagnostics. Technological advancements, including machine learning-guided patient monitoring, have reduced ICU intervention delays by 25%. Local players such as Pfizer Inc. are actively implementing immunomodulator programs across multi-center hospitals, enhancing early detection and personalized therapy. Regional consumer behavior shows higher enterprise adoption in healthcare, particularly in urban hospital networks, with over 65% of institutions integrating advanced sepsis management protocols by 2025.
What strategies are driving adoption of precision therapeutics across major healthcare hubs?
Europe accounts for 28% of the Sepsis Therapeutics Market, with Germany, UK, and France leading adoption. Regulatory frameworks such as EMA guidelines promote early-stage therapeutic trials and ensure safety compliance. Hospitals and specialized clinics are integrating rapid diagnostics and biomarker-driven therapies, improving patient response rates by 30%. Local players, including GlaxoSmithKline, are deploying immunomodulator programs across critical-care units. Consumer behavior is influenced by strict regulatory expectations, with 58% of hospitals prioritizing explainable and verifiable sepsis treatment protocols. Emerging technologies such as AI-assisted decision support and digital monitoring systems are being implemented across major European healthcare networks.
How are hospital expansions and digital health innovations reshaping patient outcomes?
Asia-Pacific holds 18% of the Sepsis Therapeutics Market by volume, with China, India, and Japan as the top consuming countries. Investment in hospital infrastructure and critical-care facilities has increased by over 35% since 2023, while innovation hubs are driving AI-assisted sepsis diagnostics adoption in urban centers. Local players such as Takeda Pharmaceuticals are conducting multi-center clinical programs to expand biologics availability. Consumer behavior is rapidly shifting toward mobile health solutions and telemedicine, with 47% of critical-care hospitals now utilizing remote monitoring to enhance early intervention and improve patient management outcomes.
What initiatives are enhancing healthcare access and therapeutic adoption in emerging markets?
South America accounts for 7% of the Sepsis Therapeutics Market, with Brazil and Argentina as leading countries. Growth is supported by government healthcare incentives and emerging hospital networks, expanding ICU coverage by 22% since 2024. Local firms are implementing training programs for sepsis management, improving early detection compliance across 60 hospitals. Infrastructure improvements, particularly in critical-care and laboratory capabilities, are driving adoption of biomarker-guided therapeutics. Consumer behavior trends indicate rising trust in hospital-administered therapies, with demand influenced by language localization and public awareness campaigns targeting sepsis management.
How is technological modernization supporting critical-care improvements in emerging healthcare hubs?
The Middle East & Africa accounts for 5% of the Sepsis Therapeutics Market, with UAE and South Africa driving regional demand. Adoption is facilitated by modernization of hospitals and digital monitoring systems, improving early sepsis detection by 18% in pilot programs. Local regulations and trade partnerships enable access to advanced biologics and immunotherapies. Regional players are investing in AI-based patient monitoring platforms to optimize ICU interventions. Consumer behavior varies, with private hospitals showing higher adoption rates while public sector uptake is growing steadily due to targeted healthcare initiatives and awareness campaigns.
United States – 42%: Dominance driven by advanced ICU infrastructure, widespread AI-assisted diagnostics, and strong hospital network adoption.
Germany – 12%: Leadership due to stringent regulatory frameworks, early implementation of biomarker-guided therapeutics, and robust hospital infrastructure.
The Sepsis Therapeutics market exhibits a moderately fragmented competitive environment, with over 120 active global players competing across biologics, antibiotics, and immunomodulator segments. The top five companies collectively account for approximately 38% of the market, reflecting strong presence yet leaving significant opportunity for mid-sized and emerging firms. Leading competitors are strategically investing in research collaborations, AI-driven diagnostics, and next-generation therapeutic development. In 2025 alone, over 35 product launches and 22 strategic partnerships were reported worldwide, focusing on precision immunotherapies and biomarker-guided sepsis interventions. Innovation trends such as AI-assisted early detection, telemedicine integration, and rapid diagnostic kits are reshaping competitive positioning. North America dominates competitive activity, contributing to 42% of regional market adoption, while Europe emphasizes regulatory-compliant, explainable therapeutics. Emerging players in Asia-Pacific and Middle East & Africa are expanding rapidly through localized clinical trials and hospital partnerships, increasing their adoption footprint. The competitive landscape is further influenced by government incentives, regulatory approvals, and ESG-aligned manufacturing initiatives, compelling companies to prioritize sustainability, efficiency, and patient-centric innovation.
GlaxoSmithKline
Novartis
AM-Pharma
Merck & Co.
Takeda Pharmaceuticals
Sanofi
Bristol-Myers Squibb
The Sepsis Therapeutics Market is increasingly driven by the integration of advanced technologies that enhance early detection, treatment precision, and patient management. AI-powered predictive algorithms are now implemented in over 58% of ICU networks across North America and Europe, reducing treatment delays by up to 28% and improving patient survival rates. Biomarker-guided therapeutics are a major technological advancement, enabling clinicians to tailor immunomodulators and antibiotics to individual patient profiles; hospitals adopting these systems report a 32% improvement in therapeutic efficacy compared to traditional broad-spectrum approaches.
Digital health platforms, including telemedicine and remote patient monitoring, are expanding rapidly in Asia-Pacific, covering 47% of critical-care facilities in 2025. These platforms enable continuous vital sign tracking, early sepsis alerts, and real-time clinician intervention, reducing emergency transfers by nearly 19%. Point-of-care rapid diagnostic tools are also transforming the market, with over 65% of leading hospitals deploying microfluidic-based sepsis tests that deliver results within two hours, accelerating therapy initiation.
Emerging technologies such as host-response immunotherapies, AI-guided dosing optimization, and integration of cloud-based electronic health records (EHR) for sepsis management are reshaping market dynamics. Localized production of next-generation biologics using automated bioreactors is improving consistency and scalability, particularly in North America and Europe. Collectively, these technologies are positioning the Sepsis Therapeutics Market to deliver higher patient outcomes, operational efficiency, and data-driven clinical decision-making while supporting sustainable and compliant healthcare practices.
• In January 2025, Partner Therapeutics, Inc. entered a collaboration with the U.S. Biomedical Advanced Research and Development Authority (BARDA) to fund a Phase 2 clinical study on sargramostim (LEUKINE) for sepsis patients, focusing on optimized dosing and immune status measurement to enhance targeted immunomodulatory therapy development.
• In 2025, deepull secured an oversubscribed €50 million Series C financing round to advance its UllCORE rapid sepsis pathogen detection system, capable of identifying 95 % of sepsis‑causing organisms and antibiotic resistance genes from blood in about one hour, moving toward broader clinical validation and regulatory pathways. (MedPath)
• In October 2025, Bluejay Diagnostics completed a $4.5 million private placement to accelerate FDA approval activities and clinical studies for its Symphony System sepsis diagnostic platform, which delivers IL‑6‑based triage results in approximately 20 minutes for near‑patient critical care use. (MedPath)
• In 2025, bioMérieux expanded its VIDAS® BRAHM S PCT early sepsis risk stratification assay and Roche Diagnostics introduced AI‑integrated sepsis alert capabilities for its cobas® Liat platforms, enhancing rapid diagnostic and clinical decision support workflows in hospital settings.
The Sepsis Therapeutics Market Report encompasses a comprehensive assessment of global therapeutic solutions used to manage and treat sepsis, addressing product segmentation, clinical applications, technology integration, and industry focus areas. It covers a wide spectrum of product categories including biologics (immunomodulators, host‑response agents), antibiotics tailored to sepsis pathogens, and supportive care therapeutics used in conjunction with standard critical‑care protocols. The report evaluates end‑user segments such as hospitals, specialty clinics, outpatient care facilities, and remote monitoring services, offering insights into adoption patterns, clinical utility, and decision‑support technologies across regions. Geographic analysis includes North America, Europe, Asia‑Pacific, South America, and Middle East & Africa, with regional data on therapeutic adoption rates, clinical trial activities, regulatory alignment, and healthcare infrastructure readiness.
Technological focus areas in the report include rapid diagnostic technologies (point‑of‑care assays, molecular panels), AI‑enabled early detection and treatment optimization tools, and integration of digital health platforms for real‑time sepsis management. The scope further highlights emerging innovations such as host‑response biomarker indexing, automated decision support systems, and advanced immunotherapies under clinical development. Detailed segmentation by therapeutic type and application allows decision‑makers to identify strategic opportunities in precision immunomodulation, pathogen‑specific antibiotic regimens, and novel supportive interventions that improve patient outcomes. The report also discusses operational influences such as regulatory pressures, clinical guideline shifts, and institutional preparedness for sepsis care in evolving healthcare ecosystems.
| Report Attribute/Metric | Report Details |
|---|---|
|
Market Revenue in 2025 |
USD V2025 Million |
|
Market Revenue in 2033 |
USD V2033 Million |
|
CAGR (2026 - 2033) |
6.7% |
|
Base Year |
2025 |
|
Forecast Period |
2026 - 2033 |
|
Historic Period |
2021 - 2025 |
|
Segments Covered |
By Types
By Application
By End-User
|
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Region Covered |
North America, Europe, Asia-Pacific, South America, Middle East, Africa |
|
Key Players Analyzed |
Pfizer Inc., GlaxoSmithKline, Johnson & Johnson, Novartis, AM-Pharma, Merck & Co., Takeda Pharmaceuticals, AstraZeneca, Sanofi, Bristol-Myers Squibb |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
