Reports
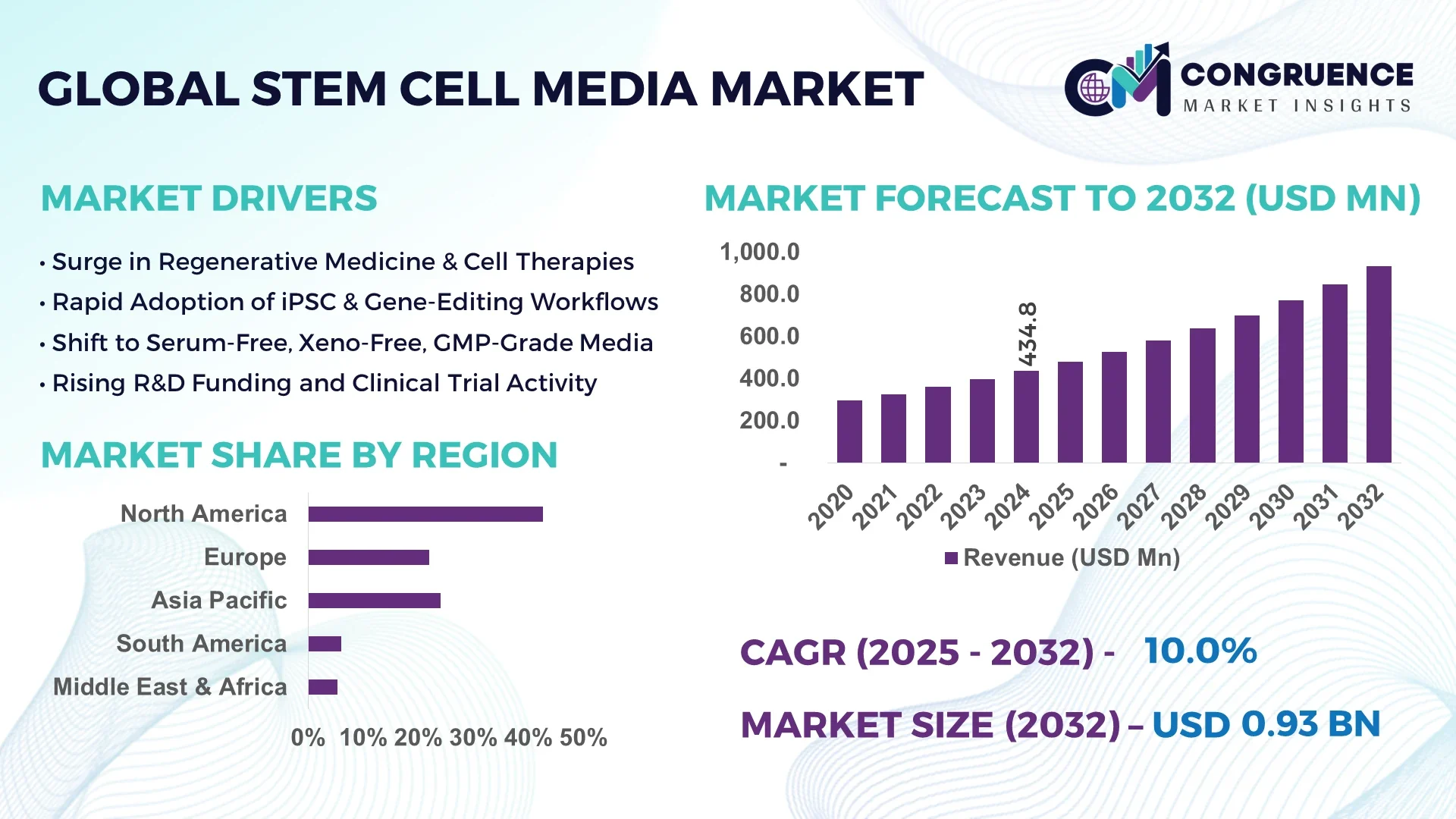
The Global Stem Cell Media Market was valued at USD 434.83 Million in 2024 and is anticipated to reach a value of USD 932.09 Million by 2032 expanding at a CAGR of 10.0% between 2025 and 2032.
The United States currently leads the Stem Cell Media Market, supported by advanced production facilities, consistent investments in regenerative medicine infrastructure, a high volume of clinical research, and the integration of automated bioprocessing systems in stem cell expansion and differentiation.
The Stem Cell Media Market is witnessing significant advancements across research, clinical, and commercial manufacturing segments, driven by increasing adoption in regenerative therapies, drug development, and disease modeling. Biopharmaceutical companies and academic institutes are key contributors to market expansion, with innovative product formulations enabling improved viability and consistency in stem cell cultures. Regulatory frameworks are evolving to support clinical-grade stem cell media production, encouraging safe scalability for therapeutic applications. The market is also shaped by advancements in serum-free and xeno-free media tailored for induced pluripotent stem cells (iPSCs) and mesenchymal stem cells (MSCs), aligning with GMP standards for downstream processing. Regional consumption patterns are influenced by the rising demand for personalized medicine and cell-based therapies, while emerging trends point to the use of automated bioreactor systems and real-time monitoring technologies that enhance reproducibility and quality control in the Stem Cell Media Market.
Artificial intelligence is transforming the Stem Cell Media Market by enabling predictive analytics, process optimization, and enhanced quality control throughout the stem cell culture lifecycle. AI-powered image analysis is now utilized for precise monitoring of stem cell morphology and confluence, reducing human error while accelerating decision-making in cell culture management. Machine learning algorithms are increasingly deployed for optimizing media formulation, allowing researchers and manufacturers to identify the most effective combinations for specific cell lines with higher precision and reduced experimental cycles. In the Stem Cell Media Market, AI-assisted systems are supporting predictive maintenance of bioreactors and environmental control systems, minimizing downtime and ensuring consistency in cell viability during scale-up operations.
Furthermore, AI models are integrated into high-throughput screening processes to evaluate the impact of different media compositions on stem cell proliferation and differentiation, streamlining the R&D pipeline for therapeutic and research applications. These AI-driven advancements in the Stem Cell Media Market contribute to reducing production costs while maintaining high-quality standards, aligning with GMP and regulatory compliance requirements in cell therapy manufacturing. The integration of AI for real-time monitoring and adaptive control in bioprocesses is fostering a new era of automated, intelligent systems capable of adjusting nutrient concentrations, pH, and oxygen levels to optimize stem cell expansion and differentiation outcomes. As the demand for scalable, high-quality stem cell products grows globally, AI is set to play a crucial role in the evolution of the Stem Cell Media Market by boosting operational efficiency, ensuring reproducibility, and shortening the development timelines in regenerative medicine and cell therapy manufacturing.
“In May 2024, an AI-powered platform developed by a biotechnology company successfully optimized the formulation of serum-free stem cell media, resulting in a 35% increase in cell proliferation rates and a 28% reduction in media consumption across large-scale production batches in the Stem Cell Media Market.”
The Stem Cell Media Market is experiencing a transformation driven by increasing adoption in regenerative medicine, advancements in stem cell research, and the emergence of serum-free and xeno-free media. Demand for consistent, GMP-compliant media formulations is rising as cell therapy manufacturing scales up globally, while biopharma firms and research institutes continue investing in automated systems for quality-controlled stem cell expansion and differentiation. Environmental considerations are prompting the development of sustainable and defined media components to reduce variability in research and clinical-grade applications. The integration of advanced bioprocessing technologies with the Stem Cell Media Market is also shaping production scalability, enabling manufacturers to maintain reproducibility and compliance with evolving regulatory standards, while supporting the global push toward personalized and precision medicine.
Expanding clinical trials in regenerative medicine are a significant driver for the Stem Cell Media Market as clinical-stage biopharma companies and academic research institutions increasingly require high-quality, consistent media formulations for therapeutic applications. More than 1,500 active clinical trials globally are investigating stem cell therapies for conditions such as cardiovascular diseases, neurodegenerative disorders, and orthopedic injuries, demanding scalable stem cell production using reliable media systems. This trend is driving investments in GMP-grade, serum-free media optimized for specific cell types, ensuring the viability, potency, and consistency of stem cells for therapeutic use. Additionally, the demand for standardized processes to meet regulatory requirements in clinical manufacturing further fuels the expansion of the Stem Cell Media Market across research and commercial supply chains.
High production and quality control complexity restrains the Stem Cell Media Market by creating operational challenges in maintaining consistent quality across large-scale production batches. Formulating defined, xeno-free, and GMP-compliant media involves advanced manufacturing processes and rigorous quality testing to ensure batch-to-batch consistency in cell viability and differentiation potential. Moreover, the need for specialized storage, transportation, and environmental control systems adds to the operational complexity and cost burden for manufacturers and research institutes. Strict regulatory compliance requirements and the need for extensive validation processes further contribute to delays in scaling up production capacity, limiting broader accessibility of high-quality media products in emerging markets within the Stem Cell Media Market.
The integration of automated bioprocessing systems presents a key opportunity for the Stem Cell Media Market by enhancing scalability and reducing manual intervention in stem cell culture processes. Automated closed systems equipped with real-time monitoring technologies and AI-powered control enable consistent environmental conditions and nutrient management, improving cell viability and reducing contamination risks. This technological shift is particularly beneficial in supporting large-scale manufacturing for clinical-grade stem cell products, aligning with GMP standards while minimizing human error and labor costs. As the demand for stem cell-based therapies grows, these automated solutions facilitate higher throughput and quality control in stem cell expansion and differentiation processes, paving the way for advanced manufacturing capabilities in the Stem Cell Media Market.
Regulatory and ethical hurdles pose a significant challenge within the Stem Cell Media Market, impacting the approval and commercialization of stem cell-derived therapies. Strict regulatory requirements across different regions, including detailed validation and compliance with GMP standards, necessitate substantial documentation, process audits, and quality control, leading to extended approval timelines for media formulations used in clinical manufacturing. Ethical concerns surrounding the sourcing and use of certain stem cell lines further complicate research and commercialization pathways, creating regional disparities in market accessibility. Additionally, evolving standards for media components and sterility requirements require continuous investment in process improvements, testing protocols, and facility upgrades, adding layers of complexity for stakeholders operating in the Stem Cell Media Market.
• Expansion of Serum-Free and Xeno-Free Media Adoption: The Stem Cell Media Market is witnessing accelerated uptake of serum-free and xeno-free formulations across clinical and research environments due to regulatory compliance needs and safety concerns associated with animal-derived components. Over 60% of newly developed clinical-stage cell therapy programs are now using xeno-free media to ensure consistent quality and reduce immunogenic risks in stem cell expansion and differentiation processes, aligning with GMP and advanced therapy manufacturing standards.
• Integration of Real-Time Monitoring Technologies: Advanced bioreactors integrated with real-time monitoring for pH, dissolved oxygen, and metabolite levels are gaining traction within the Stem Cell Media Market. These systems have demonstrated a measurable improvement in stem cell viability rates during large-scale expansion while enabling predictive adjustments in nutrient and waste management, improving overall culture efficiency. Facilities adopting real-time monitoring have reported up to a 25% increase in batch consistency in stem cell culture production.
• Automation in Stem Cell Bioprocessing: Automated closed systems for stem cell expansion and differentiation are being increasingly deployed across GMP facilities, reducing manual labor while ensuring contamination control. This trend is prominent in North America and parts of Asia-Pacific, where manufacturers have integrated automated platforms capable of handling media exchange, cell harvesting, and environmental monitoring, leading to consistent product quality across batches in the Stem Cell Media Market.
• Customizable Media Formulations for Targeted Cell Lines: Companies are focusing on developing customizable media solutions specifically tailored for iPSCs, MSCs, and hematopoietic stem cells to address targeted therapeutic needs. Custom formulations have enabled researchers to reduce experimental cycles and improve differentiation outcomes by up to 30%, providing a competitive advantage for advanced therapy development within the Stem Cell Media Market while supporting precision medicine and disease-specific cell therapy programs.
The Stem Cell Media Market segmentation includes detailed categorization by type, application, and end-user, each influencing demand dynamics and product development priorities across global markets. Product type segmentation reflects the shift toward serum-free and xeno-free media, essential for clinical-grade and research-grade stem cell cultures, while application segmentation highlights the growing adoption of stem cell media in regenerative medicine, drug discovery, and tissue engineering. End-user segmentation indicates a rising demand from biopharmaceutical companies, academic research institutions, and contract research organizations actively engaged in cell-based research and therapeutic development. This structured segmentation enables stakeholders to identify specific high-growth areas, optimize product offerings, and align investment strategies with emerging opportunities in the Stem Cell Media Market.
The Stem Cell Media Market includes serum-free media, xeno-free media, chemically defined media, classical media, and specialized supplements for cell expansion and differentiation. Serum-free media currently lead the segment due to their reduced variability and compliance with GMP requirements, ensuring higher reproducibility across batches for clinical applications. Xeno-free media represent the fastest-growing type, driven by the need to eliminate animal-derived components and align with regulatory demands for advanced therapies. Chemically defined media, while niche, play a critical role in providing precise control over cell culture conditions, essential for developing consistent stem cell-based products. Classical media formulations and specialized supplements continue to be used in research applications, contributing to niche demand, particularly in academic and early-stage research settings within the Stem Cell Media Market.
The primary application areas within the Stem Cell Media Market are regenerative medicine, drug discovery, disease modeling, and tissue engineering. Regenerative medicine holds the leading position, supported by a growing number of clinical trials for stem cell-based therapies addressing neurological, cardiovascular, and orthopedic conditions. Drug discovery represents the fastest-growing application, as pharmaceutical companies integrate stem cell models into preclinical testing to improve the accuracy of drug screening and toxicity studies. Disease modeling using stem cells continues to gain traction, providing platforms for studying disease mechanisms and testing targeted interventions. Tissue engineering, while a smaller segment, is expanding as engineered tissues require precise stem cell expansion using advanced media formulations, contributing to innovation in personalized medicine and advanced therapeutic development within the Stem Cell Media Market.
Key end-user segments in the Stem Cell Media Market include biopharmaceutical companies, academic and research institutes, contract research organizations, and hospitals and clinics engaged in regenerative medicine practices. Biopharmaceutical companies are the leading end-users, driven by increasing investments in cell-based therapy development and GMP-grade manufacturing capabilities requiring high-quality, consistent media solutions. Contract research organizations represent the fastest-growing end-user segment, as outsourcing of stem cell research and manufacturing processes rises globally to optimize costs and accelerate project timelines. Academic and research institutes continue to contribute significantly to the market landscape, focusing on fundamental research and pilot studies that require research-grade media formulations. Hospitals and clinics are gradually adopting stem cell-based treatments in controlled environments, contributing to niche but steady demand within the Stem Cell Media Market.
North America accounted for the largest market share at 42.7% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 12.3% between 2025 and 2032.
The Stem Cell Media Market in North America benefits from a robust network of advanced therapy manufacturers, a high concentration of clinical trials, and well-established regulatory pathways supporting GMP-compliant cell therapy production. Asia-Pacific’s rapid growth is driven by increasing investments in regenerative medicine infrastructure, rising healthcare expenditure, and growing demand for cell-based therapies in emerging economies like China and India. Europe continues to maintain steady market expansion, supported by government-backed research initiatives and the presence of a skilled biomedical workforce, while South America and the Middle East & Africa demonstrate emerging demand driven by healthcare modernization and public-private partnerships in cell-based therapy development within the Stem Cell Media Market.
Driving Cell-Based Innovation through Advanced Therapeutics Expansion
North America holds a commanding 42.7% share in the Stem Cell Media Market, driven by strong demand from biopharmaceutical companies and cell therapy developers advancing regenerative medicine pipelines. Industries such as advanced therapy medicinal products (ATMPs) and precision medicine are primary drivers of demand, utilizing GMP-grade serum-free and xeno-free media for scalable stem cell expansion. Regulatory changes, including fast-track approvals for cell-based therapies, support consistent market growth by streamlining clinical translation processes. Technological advancements, including AI-integrated bioreactor systems and automated closed-system bioprocessing, are accelerating digital transformation within the Stem Cell Media Market, ensuring improved consistency and scalability for manufacturers across the region.
Accelerating Regenerative Medicine Adoption with Technological Innovation
Holding a 29.4% share in the Stem Cell Media Market, Europe demonstrates consistent demand driven by advanced therapy adoption across Germany, the UK, and France. These key European markets leverage regenerative medicine advancements to support growing clinical and research requirements for stem cell expansion and differentiation. Regulatory bodies are fostering the use of GMP-compliant, xeno-free media by updating ATMP frameworks while incentivizing sustainable and safe cell therapy development. Adoption of emerging technologies, including AI-driven culture monitoring systems and automated media formulation platforms, enhances scalability and product consistency in the Stem Cell Media Market across Europe, aligning with regional sustainability initiatives and clinical application demand.
Emerging as a Dynamic Hub for Regenerative Medicine Manufacturing
Asia-Pacific ranks as the fastest-growing region in the Stem Cell Media Market, with China, India, and Japan leading in consumption volume and manufacturing capacity for stem cell media products. The region’s growth is driven by infrastructure investments in biopharmaceutical manufacturing and the expansion of stem cell banks to meet personalized medicine and regenerative therapy demand. Japan’s advancements in iPSC applications and China’s focus on scaling GMP-compliant cell therapy facilities contribute to consistent growth momentum. Regional tech trends include the adoption of automated bioprocessing and digital monitoring systems within cell culture workflows, supporting reproducibility and reducing operational costs for manufacturers in the Stem Cell Media Market.
Strengthening Clinical Research Ecosystems for Advanced Therapies
In South America, Brazil and Argentina are the leading contributors within the Stem Cell Media Market, with Brazil accounting for over 58% of the regional demand. The region benefits from an expanding network of regenerative medicine clinics and a growing clinical research ecosystem requiring consistent, high-quality media formulations for cell therapy applications. Infrastructure trends include the establishment of cell therapy labs and biopharmaceutical manufacturing facilities supported by regional healthcare modernization initiatives. Government incentives and supportive trade policies further enhance market accessibility, allowing the Stem Cell Media Market in the region to cater to local clinical requirements while facilitating controlled-scale manufacturing.
Advancing Stem Cell Research through Targeted Investments
In the Middle East & Africa, rising demand for advanced therapies in the UAE and South Africa is shaping growth within the Stem Cell Media Market. The region is witnessing modernization trends across healthcare systems, with investments in research laboratories and biomanufacturing facilities focused on regenerative medicine and cell therapy. Local regulations are gradually aligning with global GMP standards, ensuring the safe development and application of cell-based therapies. Trade partnerships and government-backed initiatives encourage collaboration with international biopharmaceutical firms, supporting the adoption of innovative bioprocessing systems and high-quality stem cell media for scalable, compliant production in the Stem Cell Media Market.
United States – 38.9% Market Share: The United States leads the Stem Cell Media Market due to high production capacity and robust demand for GMP-grade stem cell media across advanced therapy development pipelines.
China – 16.4% Market Share: China ranks second in the Stem Cell Media Market, supported by strong end-user demand in regenerative medicine and government-led investments in cell therapy manufacturing infrastructure.
The Stem Cell Media Market is characterized by a competitive environment with over 85 active global and regional manufacturers offering diverse media formulations for stem cell expansion and differentiation. Competition is driven by the need for GMP-compliant, serum-free, and xeno-free products that ensure batch-to-batch consistency for clinical and research applications. Companies are strategically investing in new product launches and specialized media formulations tailored for specific stem cell lines, including iPSCs and MSCs, to gain differentiation in the market. Strategic partnerships with academic institutions and biopharmaceutical firms are increasingly common to enhance product validation for clinical trials and large-scale manufacturing. Mergers and acquisitions within the market continue to reshape the landscape, with several players expanding their production capacities through facility upgrades and automation integration. Innovation trends, including AI-powered bioprocess optimization and real-time monitoring systems within automated bioreactors, are influencing the competitive dynamics by improving product quality and operational efficiency. Additionally, regional players are focusing on expanding GMP-certified manufacturing capabilities to meet rising global demand, further intensifying competition within the Stem Cell Media Market.
Thermo Fisher Scientific
Merck KGaA
Lonza Group AG
STEMCELL Technologies
Corning Incorporated
FUJIFILM Irvine Scientific
Miltenyi Biotec
Bio-Techne Corporation
Sartorius AG
PromoCell GmbH
Technological advancements in the Stem Cell Media Market are reshaping manufacturing and scalability, ensuring consistent quality and compliance for research and clinical applications. The adoption of serum-free and xeno-free media formulations is driven by regulatory and safety requirements, supporting reliable cell expansion and differentiation while reducing variability across production batches. Advanced bioreactors equipped with real-time monitoring of pH, dissolved oxygen, and nutrient levels enable optimized cell culture conditions, minimizing contamination risks and enhancing cell viability. These bioreactor systems, often integrated with AI-powered predictive analytics, facilitate dynamic adjustments to media compositions based on metabolic needs, improving yield consistency during large-scale manufacturing.
Automated closed-system bioprocessing technologies are gaining traction in GMP facilities, supporting the transition from manual processes to fully automated workflows for media exchange, environmental control, and harvesting. Single-use bioprocessing systems are also being deployed to reduce cleaning validation requirements and contamination risks while increasing operational flexibility. Additionally, customizable media formulations tailored to specific stem cell types, including iPSCs and MSCs, allow manufacturers to address targeted therapeutic applications efficiently. Technologies focusing on the development of chemically defined and animal component-free media are advancing the Stem Cell Media Market by aligning with personalized medicine trends and emerging cell therapy pipelines. These innovations collectively contribute to scalability, operational efficiency, and consistency in producing high-quality stem cell products globally.
• In February 2023, Thermo Fisher Scientific launched a new xeno-free, chemically defined stem cell media designed for iPSC expansion, enabling consistent cell quality and reducing variability during clinical-scale manufacturing while supporting advanced therapy production pipelines.
• In May 2023, Merck KGaA completed the expansion of its stem cell media manufacturing facility in Darmstadt, increasing its production capacity by 45% to meet rising demand for GMP-compliant media formulations across regenerative medicine and research applications.
• In March 2024, Lonza introduced an AI-enabled bioprocess monitoring platform integrated with its stem cell media systems, allowing real-time nutrient tracking and automated adjustments that improved stem cell viability rates by up to 28% during large-scale production.
• In April 2024, STEMCELL Technologies launched a customized media formulation service for personalized stem cell therapy developers, offering tailored media blends optimized for specific stem cell lines, reducing experimental cycles and improving differentiation outcomes for clients globally.
The Stem Cell Media Market Report comprehensively analyzes market segments including serum-free media, xeno-free media, chemically defined media, and specialized supplements, providing insight into their respective roles in supporting stem cell research, clinical trials, and large-scale manufacturing. The report covers key applications in regenerative medicine, drug discovery, disease modeling, and tissue engineering, reflecting their diverse utilization of advanced stem cell media for therapeutic and research purposes. Geographic coverage includes North America, Europe, Asia-Pacific, South America, and the Middle East & Africa, capturing regional market dynamics, infrastructure readiness, and demand patterns across each area.
Additionally, the report details technological innovations impacting the Stem Cell Media Market, including automated closed-system bioprocessing, AI-integrated bioreactors, and real-time monitoring technologies that are advancing operational efficiency and quality consistency. It explores trends toward GMP-compliant, serum-free, and xeno-free formulations while addressing the role of customizable media for targeted cell lines such as iPSCs and MSCs. The report evaluates the competitive landscape by profiling major manufacturers, analyzing their strategic initiatives, and monitoring new product developments and facility expansions. It also considers emerging niches, including personalized medicine and sustainable media development, ensuring stakeholders understand evolving opportunities within the global Stem Cell Media Market.
| Report Attribute/Metric | Report Details |
|---|---|
|
Market Revenue in 2024 |
USD 434.83 Million |
|
Market Revenue in 2032 |
USD 932.09 Million |
|
CAGR (2025 - 2032) |
10% |
|
Base Year |
2024 |
|
Forecast Period |
2025 - 2032 |
|
Historic Period |
2020 - 2024 |
|
Segments Covered |
By Types
By Application
By End-User
|
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Region Covered |
North America, Europe, Asia-Pacific, South America, Middle East, Africa |
|
Key Players Analyzed |
Thermo Fisher Scientific, Merck KGaA, Lonza Group AG, STEMCELL Technologies, Corning Incorporated, FUJIFILM Irvine Scientific, Miltenyi Biotec, Bio-Techne Corporation, Sartorius AG, PromoCell GmbH |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
