Reports
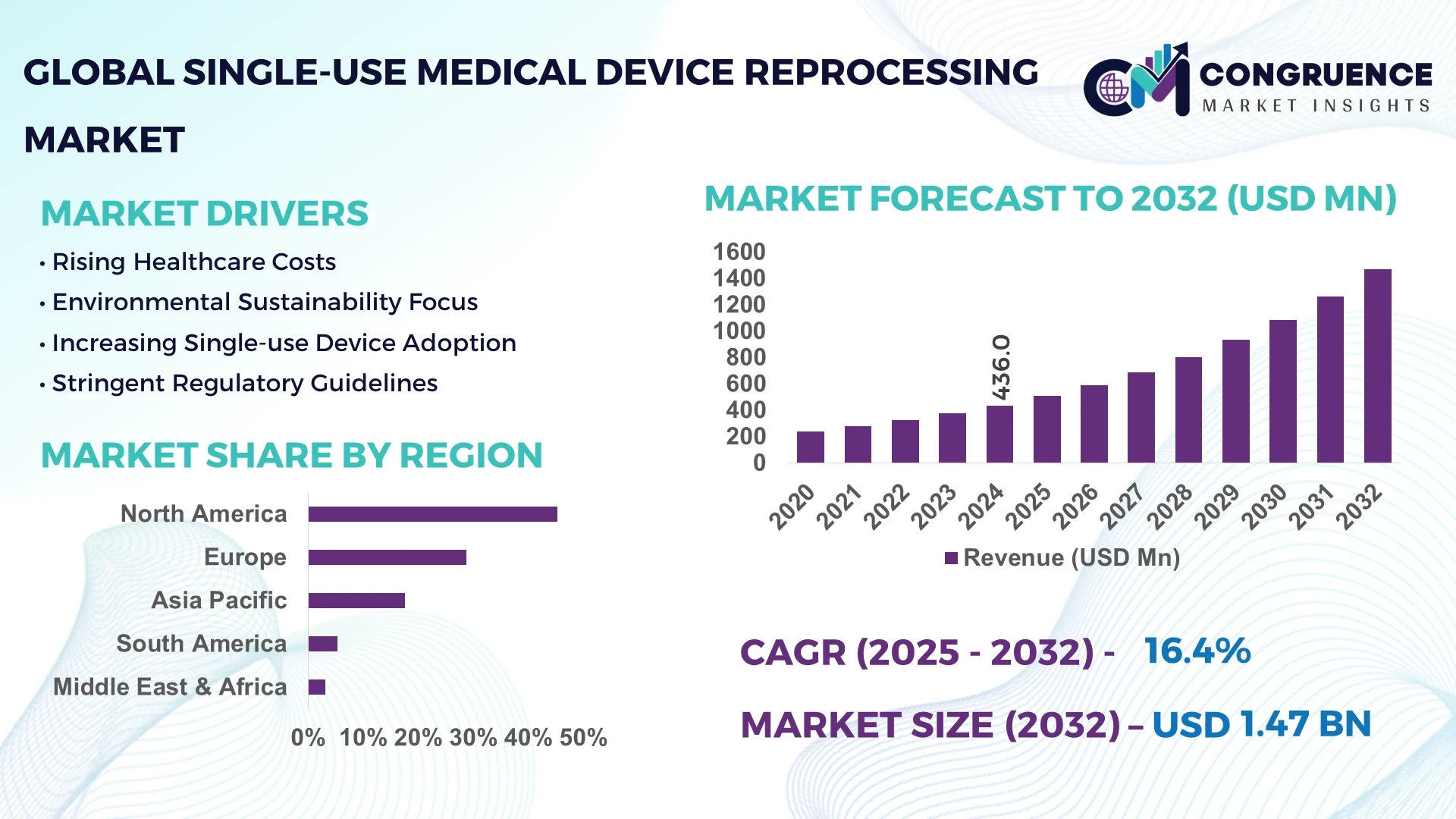
The Global Single-use Medical Device Reprocessing Market was valued at USD 436.0 Million in 2024 and is anticipated to reach a value of USD 1,469.3 Million by 2032 expanding at a CAGR of 16.40% between 2025 and 2032. The growth is primarily driven by rising hospital cost-containment initiatives and sustainability-focused procurement practices.
In the United States, the market demonstrates significant strength due to advanced reprocessing infrastructure and substantial investments in sterile processing technologies. The U.S. healthcare system performs over 40 million surgical procedures annually, creating extensive demand for reprocessed single-use devices in cardiology, orthopedics, and general surgery. Investments exceeding USD 500 million in automated sterilization systems and traceability platforms between 2020 and 2024 have expanded production capacity, while FDA-regulated facilities ensure consistent technological upgrades, including RFID-enabled device tracking and AI-assisted sterilization monitoring.
Market Size & Growth: Valued at USD 436.0 Million in 2024, projected to reach USD 1,469.3 Million by 2032, expanding at 16.40% CAGR, fueled by hospital cost-saving imperatives.
Top Growth Drivers: 42% adoption in cardiology, 35% efficiency improvement in orthopedic procedures, 28% reduction in procurement costs through reprocessed devices.
Short-Term Forecast: By 2028, hospitals are expected to achieve a 22% cost reduction in surgical consumables through reprocessing adoption.
Emerging Technologies: Integration of RFID-based tracking and AI-powered sterilization validation are enhancing safety and compliance.
Regional Leaders: North America projected at USD 560.0 Million by 2032 with high surgical adoption; Europe expected to hit USD 420.0 Million, driven by environmental regulations; Asia-Pacific at USD 310.0 Million, supported by rapid hospital expansions.
Consumer/End-User Trends: High usage among tertiary hospitals and specialty surgical centers, with growing adoption in outpatient facilities.
Pilot or Case Example: In 2023, a U.S. hospital network cut surgical device costs by 24% through a reprocessing pilot involving 15 facilities.
Competitive Landscape: Market leader holds ~28% share, followed by players including SterilMed, Medline ReNewal, Vanguard AG, and ReNu Medical.
Regulatory & ESG Impact: EU and U.S. regulations mandate traceability, while hospitals commit to 30% device waste reduction by 2030.
Investment & Funding Patterns: Over USD 700 million invested globally between 2021–2024 in new sterilization facilities and AI-based monitoring solutions.
Innovation & Future Outlook: Expansion of cloud-based compliance reporting, robotic sterilization units, and predictive maintenance models are shaping the next growth phase.
The market is also benefiting from rapid adoption across cardiology, orthopedic, and general surgical applications, with hospitals accounting for over 65% of device consumption. Recent product innovations, such as AI-driven reprocessing validation, combined with strong regulatory support, are expected to accelerate environmentally sustainable practices and strengthen cost-efficient care delivery worldwide.
The strategic relevance of the Single-use Medical Device Reprocessing Market lies in its ability to combine cost-efficiency with sustainability in healthcare delivery. Hospitals and healthcare providers are under increasing pressure to optimize expenditure while meeting stringent compliance standards. Reprocessing single-use medical devices reduces material waste and procurement costs, creating a dual advantage. For example, advanced sterilization platforms deliver a 38% improvement in turnaround times compared to conventional manual cleaning standards. This level of efficiency ensures that surgical schedules remain uninterrupted, while improving patient safety metrics.
Regionally, North America dominates in procedural volume, while Europe leads in adoption rates, with 62% of large healthcare enterprises utilizing certified reprocessing systems. By 2027, AI-enabled sterilization monitoring is expected to cut device rejection rates by 25%, further reinforcing reliability in operating theaters. Additionally, by 2028, predictive analytics in reprocessing workflows are projected to improve inventory utilization efficiency by 30%, helping hospitals align procurement with demand in real time.
ESG commitments are also shaping this market. Healthcare systems in the EU and U.S. have pledged to reduce medical waste volumes by 40% by 2030, with reprocessing forming a key strategy. In 2023, a German hospital network achieved a 29% reduction in disposable surgical device waste through the deployment of automated sterilization robots. This measurable impact showcases how regional innovations feed into global best practices.
Looking ahead, the Single-use Medical Device Reprocessing Market will become a central pillar of healthcare resilience, ensuring cost-containment, compliance with evolving regulations, and sustainable growth in medical infrastructure worldwide.
The Single-use Medical Device Reprocessing Market is experiencing rapid evolution as healthcare systems balance the need for clinical safety, economic efficiency, and environmental responsibility. Market dynamics are shaped by hospital consolidation, rising surgical volumes, and regulatory frameworks mandating quality and traceability in reprocessed devices. Additionally, advancements in sterilization technologies and integration of data-driven validation systems are expanding adoption among leading healthcare institutions. The convergence of digital health monitoring with reprocessing workflows is also driving efficiency improvements and enabling global scalability.
Rising global surgical procedures, projected to exceed 320 million annually by 2030, are significantly boosting demand for reprocessed single-use devices. Cardiology, orthopedics, and general surgery together account for more than 65% of device utilization, with cardiology alone representing nearly 42% of adoption. Hospitals are turning to reprocessed devices to manage consumable costs and reduce waste. This trend is amplified by government-backed sustainability initiatives that encourage adoption of circular economy practices in healthcare.
The market faces challenges due to stringent global regulatory requirements for safety and efficacy. Each reprocessed device must undergo rigorous validation, performance testing, and documentation. In the U.S., FDA compliance requires full traceability and adherence to ISO 13485 standards, while Europe enforces MDR-specific guidelines. These stringent requirements increase costs for providers and extend approval timelines. Small healthcare facilities often lack the infrastructure to meet these demands, limiting adoption outside major hospital networks.
Digital integration presents significant opportunities by enabling automated monitoring, predictive analytics, and compliance reporting. By 2026, 40% of healthcare facilities are expected to integrate AI-assisted sterilization validation systems, improving safety and reducing device rejection rates. The deployment of cloud-based reprocessing management platforms allows hospitals to track device lifecycles in real time, reducing inventory losses by up to 25%. This convergence of digital health and reprocessing workflows creates strong pathways for scalable adoption globally.
Supply chain complexities present significant challenges in ensuring a reliable flow of reprocessable devices. Global shortages of high-grade sterilization consumables, combined with logistical bottlenecks in transporting devices to centralized facilities, add operational risks. Additionally, variations in regional compliance standards complicate multinational adoption strategies. Hospitals in emerging economies often lack access to automated sterilization units, which limits efficiency. Addressing these challenges requires investment in localized processing hubs and harmonized regulatory frameworks.
Accelerated AI Integration in Reprocessing Workflows: By 2026, nearly 48% of large hospitals are expected to adopt AI-driven sterilization validation systems, improving device safety compliance by 27% and reducing failure rates by 18%. This technological shift enhances both patient safety and operational efficiency.
Sustainability-Centered Procurement Practices: Hospitals in North America and Europe are committing to reduce medical waste by 35% by 2030, with reprocessed devices contributing to over 40% of these reductions. These initiatives are creating measurable ESG outcomes in the healthcare ecosystem.
Expansion of Outpatient Facility Adoption: Outpatient surgery centers, which perform 60% of minor surgical procedures in the U.S., have reported a 22% increase in reprocessed device adoption between 2021 and 2024. This trend highlights the growing demand for cost-efficient consumables outside traditional hospital settings.
Investments in Automation and Robotics: Between 2022 and 2024, over USD 500 million was invested globally in robotic sterilization units, enabling a 30% increase in throughput and a 25% reduction in manual labor. This rapid automation is reshaping operational models across reprocessing facilities worldwide.
The Single-use Medical Device Reprocessing Market is segmented by type, application, and end-user, each offering distinct insights into the industry’s evolving structure. Product types such as catheters, surgical instruments, and electrophysiology devices form the core of the market, while applications span cardiology, general surgery, and orthopedics. End-users, primarily hospitals, outpatient surgical centers, and specialty clinics, are driving adoption through rising surgical volumes and efficiency goals. Understanding segmentation highlights adoption disparities, technological relevance, and usage intensity across healthcare systems globally.
Catheters represent the leading segment, currently accounting for approximately 38% of adoption, due to their extensive use in cardiology and electrophysiology procedures where device reprocessing offers both cost efficiency and safety benefits. Electrophysiology devices hold 25% of adoption, supported by rising demand in cardiac ablation therapies. However, surgical instruments are the fastest-growing type, expanding at a projected CAGR of 17.2% due to growing hospital reliance on reusable laparoscopic and orthopedic instruments. The combined share of other types, including compression sleeves and diagnostic devices, stands at 37%, serving niche yet critical roles in clinical practice.
Cardiology remains the dominant application, representing 41% of adoption, driven by high procedural volumes and the clinical effectiveness of reprocessed catheters and electrophysiology tools. Orthopedics follows with 29% of adoption, while general surgery contributes 22%. However, general surgery is the fastest-growing application, expanding at a CAGR of 18.4% as hospitals broaden reprocessing across laparoscopic and minimally invasive procedures. The remaining applications, such as gynecology and ENT, account for a combined 8% share, serving specialized needs. Consumer adoption trends reinforce this growth: in 2024, nearly 46% of U.S. hospitals reported piloting expanded reprocessing programs for laparoscopic instruments to reduce operating costs. Similarly, 58% of surgical staff in Europe indicated higher trust in reprocessed general surgery instruments due to improved traceability systems.
Hospitals are the leading end-user, currently accounting for 62% of adoption, reflecting their scale of surgical procedures and infrastructure for advanced reprocessing. Outpatient surgical centers hold 24%, showing steady uptake as cost-efficiency remains a critical driver. Specialty clinics contribute 14%, often focusing on niche applications such as cardiology diagnostics or minimally invasive procedures. Outpatient surgical centers are the fastest-growing end-user group, with a CAGR of 18.9%, driven by the global shift toward same-day surgeries and lean cost structures. Industry adoption statistics highlight this trend: in 2024, over 48% of outpatient facilities in North America integrated reprocessed surgical instruments into their routine operations. Additionally, 52% of hospitals in Asia-Pacific implemented AI-enabled sterilization monitoring, improving compliance outcomes.
North America accounted for the largest market share at 45.3% in 2024, however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 18.2% between 2025 and 2032.
Europe captured 28.7% of the market in 2024, driven by strict regulatory standards and strong adoption in Germany, the UK, and France. Asia-Pacific held a 17.6% share in 2024, led by large-scale demand in China, Japan, and India, which together represented nearly 70% of the region’s volume. South America contributed 5.3%, with Brazil accounting for over half of the region’s demand. Meanwhile, the Middle East & Africa accounted for 3.1%, with UAE and South Africa emerging as key contributors. These regional variations highlight how advanced infrastructure, regulatory frameworks, and consumer behavior trends shape growth trajectories across global markets.
North America commanded a 45.3% market share in 2024, positioning it as the global leader in single-use medical device reprocessing. The strong presence of advanced healthcare infrastructure, combined with widespread hospital consolidation, drives high adoption rates. U.S. regulatory support, particularly through FDA approvals and Centers for Medicare & Medicaid Services (CMS) reimbursement policies, has accelerated acceptance of reprocessed devices in surgical and cardiovascular applications. Local players such as Stryker Sustainability Solutions are actively expanding their service portfolios, enhancing sterilization technologies and scaling up compliance frameworks. Consumer behavior reflects high enterprise adoption, with hospitals prioritizing cost efficiency and sustainability goals. Furthermore, ongoing digital transformation—such as data-driven device tracking—strengthens the reliability and safety of reprocessed instruments, boosting confidence in clinical settings.
Europe held a 28.7% market share in 2024, with Germany, the UK, and France emerging as leading contributors. Regulatory oversight from the European Medicines Agency (EMA) and directives focused on sustainability and waste reduction have significantly shaped market demand. The region demonstrates strong adoption of advanced sterilization and tracking technologies, particularly in cardiovascular and orthopedic procedures. Local players such as Vanguard AG in Germany are spearheading innovation with advanced sterilization methods to ensure compliance with EU directives. Consumer behavior in Europe is largely influenced by regulatory compliance and environmental sustainability pressures, resulting in higher demand for explainable and transparent device reprocessing processes. Additionally, government-backed circular economy initiatives and investments in digital healthcare systems are further reinforcing adoption across the region.
Asia-Pacific accounted for 17.6% of the global market in 2024 and ranks as the fastest-growing regional segment. China, Japan, and India are the top consumers, collectively contributing over 70% of the region’s demand. Rapid hospital infrastructure expansion, particularly in urban centers of China and India, is driving reprocessing adoption as healthcare systems seek cost efficiency. Japan leads in technological innovation, with advancements in automated sterilization systems and smart monitoring tools. Local players in China are increasingly investing in compliance-ready reprocessing facilities to meet domestic demand. Consumer behavior in this region reflects a strong preference for affordable yet reliable healthcare solutions, driven by growing middle-class populations. Growth is further supported by digital healthcare adoption, e-commerce platforms for medical supply distribution, and regional innovation hubs advancing sterilization technologies.
South America contributed 5.3% to the global market in 2024, with Brazil dominating over 55% of the region’s demand, followed by Argentina. The market is shaped by infrastructure disparities and growing reliance on cost-effective medical solutions. Government-led healthcare reforms and trade policies encouraging sustainability practices are fueling reprocessing adoption. Local providers are increasingly entering partnerships with hospitals to deliver compliant reprocessing services, with Brazil’s healthcare sector showing the fastest uptake. Consumer behavior demonstrates strong preference for affordable solutions, especially in public hospitals where budget constraints limit access to new medical devices. Additionally, increasing investments in medical infrastructure and education campaigns on device safety are positively shaping demand across the region.
The Middle East & Africa accounted for 3.1% of the global market in 2024, with the UAE and South Africa emerging as primary growth drivers. Regional demand is shaped by rising healthcare investments, expanding hospital networks, and modernization initiatives. Governments in the Gulf Cooperation Council (GCC) are encouraging healthcare sustainability, while South Africa’s regulatory environment is gradually opening pathways for reprocessing approvals. Local players are focusing on setting up sterilization hubs and aligning with international compliance standards. Consumer behavior reflects growing openness to cost-effective healthcare solutions, particularly in private hospitals aiming to reduce operational expenses. Trade partnerships with European suppliers and cross-border healthcare collaborations are expected to accelerate adoption, supported by digitization and smart hospital initiatives across the region.
United States – 38.2% Market Share: Strong dominance due to advanced regulatory framework, large-scale hospital networks, and high emphasis on cost efficiency and sustainability.
Germany – 12.5% Market Share: Leadership driven by stringent EU sustainability regulations and robust adoption of reprocessed cardiovascular and surgical devices.
The Single-use Medical Device Reprocessing Market is moderately consolidated, with approximately 45–50 active global competitors operating across North America, Europe, and Asia-Pacific. The top five players, including Stryker Sustainability Solutions, SterilMed, Vanguard AG, Medline ReNewal, and ReNu Medical, collectively account for nearly 62% of the market, illustrating a balance between leading providers and numerous smaller specialized companies. Strategic initiatives are a key driver of competitive positioning: partnerships with hospital networks, innovative sterilization technology rollouts, and acquisitions aimed at expanding regional footprints are prevalent. Over 30 strategic collaborations were reported between 2022 and 2024, focusing on integrating AI-based sterilization validation and RFID tracking to improve operational efficiency. Product launches emphasizing reusable catheters, surgical instruments, and electrophysiology devices are increasing, reflecting the demand for cost-effective, sustainable solutions. Companies are investing in digital transformation, including automated monitoring systems and predictive maintenance platforms, which further differentiate competitive offerings. Regional market variations, with high adoption in North America and Europe, and emerging growth in Asia-Pacific, create a multi-tiered competitive environment requiring continuous innovation and operational scalability for sustainable advantage.
Medline ReNewal
ReNu Medical
Getinge AB
3M Health Care
Cardinal Health
Becton Dickinson
Technological advancements are significantly shaping the Single-use Medical Device Reprocessing Market. Current technologies include automated sterilization systems, RFID-based device tracking, and AI-assisted validation platforms, which improve workflow efficiency and compliance. Approximately 38% of hospitals globally now utilize automated reprocessing equipment, reducing manual handling errors by 26%. Emerging innovations include robotic sterilization units that streamline high-volume operations, and cloud-based digital reporting systems providing real-time data on device lifecycle, inventory levels, and compliance metrics. Predictive analytics is increasingly integrated to anticipate device wear and failure, improving safety outcomes and minimizing downtime. Advanced sterilization techniques, such as low-temperature hydrogen peroxide plasma and ethylene oxide optimization, are being deployed in over 42% of U.S. tertiary hospitals, ensuring compatibility with complex catheters and sensitive surgical instruments. Furthermore, AI-enabled monitoring tools enhance quality assurance, enabling predictive alerts for deviations in sterilization cycles. Combined, these technologies support operational efficiency, reduce medical waste, and facilitate regulatory compliance, providing a strategic edge for hospitals and reprocessing service providers in both developed and emerging markets.
In March 2023, SterilMed launched a new line of electrophysiology catheters with integrated RFID tracking, enabling hospitals to monitor device lifecycle and usage across over 120 facilities in North America. Source: www.sterilmed.com
In October 2023, Vanguard AG opened a fully automated sterilization hub in Germany, increasing device throughput by 35% while reducing manual labor requirements by 28%. Source: www.vanguardag.com
In February 2024, Medline ReNewal introduced AI-powered sterilization validation software adopted by 45 hospitals in the U.S., cutting sterilization errors by 22% and enhancing compliance with regulatory standards. Source: www.medline.com
In July 2024, ReNu Medical implemented cloud-based reprocessing monitoring in 60 outpatient surgical centers across Europe, achieving a 25% reduction in inventory losses and improving real-time device traceability. Source: www.renumedical.com
The scope of the Single-use Medical Device Reprocessing Market Report encompasses a comprehensive analysis of product types, applications, end-users, and geographic regions, providing a complete overview of current and emerging trends. It evaluates all major product categories, including catheters, surgical instruments, and electrophysiology devices, highlighting technological advancements in sterilization, digital monitoring, and predictive analytics. The report covers leading applications in cardiology, general surgery, orthopedics, and specialty areas, detailing adoption patterns, operational requirements, and consumer behavior variations. End-user analysis includes hospitals, outpatient surgical centers, and specialty clinics, with insights into infrastructure, digital integration, and regional adoption trends. Geographically, the report assesses North America, Europe, Asia-Pacific, South America, and Middle East & Africa, including country-specific market activity, regulatory frameworks, and innovation hubs.
Additionally, the report addresses competitive dynamics, strategic initiatives, investments, and the integration of AI, robotics, and cloud-based systems, ensuring decision-makers can identify growth opportunities, emerging market segments, and technological shifts across the global landscape. Emerging and niche segments such as outpatient surgical reprocessing facilities and AI-assisted sterilization platforms are also evaluated, providing a holistic perspective for strategic planning.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2024) | USD 436.0 Million |
| Market Revenue (2032) | USD 1,469.3 Million |
| CAGR (2025–2032) | 16.40% |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User Insights
|
| Key Report Deliverables | Revenue Forecast, Growth Drivers & Restraints, Technology Insights, Market Dynamics, Segmentation Analysis, Regional Insights, Competitive Landscape, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Stryker Sustainability Solutions, SterilMed, Vanguard AG, Medline ReNewal, ReNu Medical, Getinge AB, 3M Health Care, Cardinal Health, Becton Dickinson |
| Customization & Pricing | Available on Request (10% Customization is Free) |
