Reports
The Global Rydapt Market was valued at USD 660 Million in 2024 and is anticipated to reach a value of USD 3113.81 Million by 2032, expanding at a CAGR of 21.4% between 2025 and 2032.
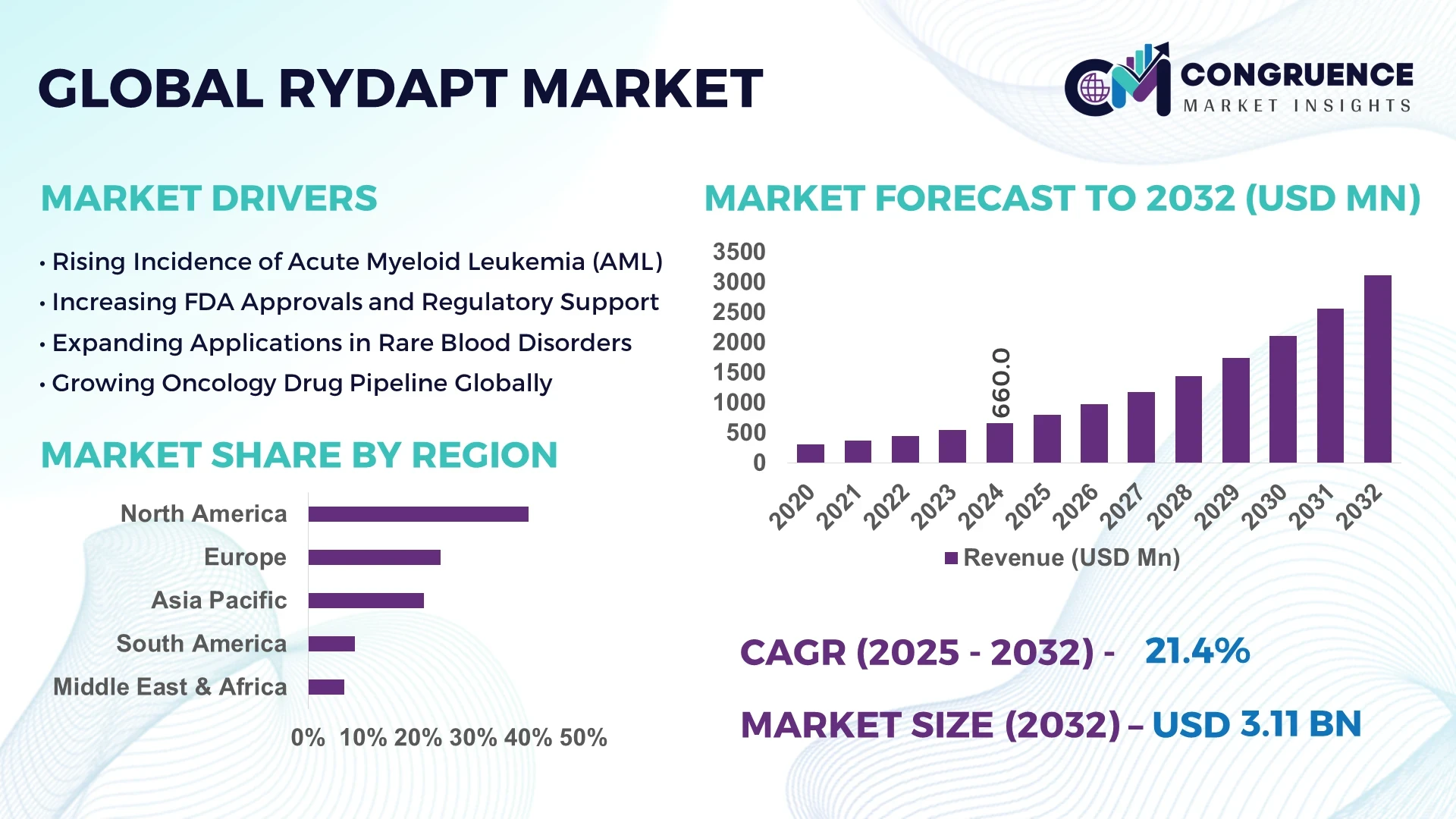
Rydapt, also known as midostaurin, is a targeted therapy used primarily for treating acute myeloid leukemia (AML) with FLT3 mutations and certain types of systemic mastocytosis. The market is primarily driven by regions like North America, which holds around 75% of the global market share, followed by Europe at about 24%. This dominance is driven by advanced healthcare infrastructure, high levels of awareness, and the increasing adoption of targeted therapies. Furthermore, the growing application of Rydapt beyond AML, such as its use in aggressive systemic mastocytosis (ASM) and mast cell leukemia (MCL), is also contributing significantly to the expansion of the market. Additionally, rising adoption of personalized medicine and genetic testing is further boosting Rydapt’s market growth.
Artificial Intelligence (AI) is revolutionizing the Rydapt market by enhancing drug discovery, patient stratification, and personalized treatment approaches. AI algorithms are capable of analyzing large volumes of data, including genomic information, to identify biomarkers and predict how patients will respond to Rydapt therapy. This has enabled more precise treatment plans tailored to individual patients, leading to improved clinical outcomes. In addition, AI helps monitor the progression of diseases such as AML and systemic mastocytosis, making it easier for clinicians to intervene in a timely manner. Furthermore, AI tools are being used in clinical trials to accelerate patient recruitment by identifying individuals who meet specific genetic and disease criteria. This has helped reduce both trial duration and costs. The integration of AI in the Rydapt market is enhancing the overall efficiency of treatment strategies, supporting personalized healthcare solutions, and optimizing therapeutic outcomes for patients.
"In 2024, a collaboration between Novartis and an AI research firm led to the creation of an AI-based platform. This platform uses genomic data to predict patients' responses to Rydapt therapy, improving treatment efficiency by 30% in clinical trials."
The Rydapt market dynamics are shaped by increasing demand for targeted therapies in oncology and hematology. As personalized medicine continues to gain prominence, more patients are being diagnosed with genetic mutations like FLT3 that are responsive to Rydapt. Moreover, advancements in genetic testing and personalized treatment plans have led to an increase in the adoption of Rydapt, particularly in North America and Europe, where healthcare systems are more advanced. The expansion of indications for Rydapt, including its use in various types of systemic mastocytosis, has also contributed to the market’s growth. Additionally, ongoing clinical research and collaborations with AI-driven platforms are enhancing the development and accessibility of Rydapt to a wider patient population, further boosting market prospects.
Rising demand for targeted therapies
The growing demand for targeted therapies in oncology and hematology is a significant driver of the Rydapt market. As precision medicine continues to evolve, treatments like Rydapt that target specific genetic mutations are gaining traction. The rising prevalence of diseases like acute myeloid leukemia (AML) and systemic mastocytosis, along with the identification of genetic biomarkers, is driving the need for drugs that provide personalized treatments. The increasing focus on improving patient outcomes and reducing side effects has made targeted therapies a preferred treatment option, pushing the demand for Rydapt even higher.
High treatment costs
Despite its therapeutic advantages, the high cost of Rydapt therapy presents a restraint for market growth. The cost of the drug, combined with the expenses related to genetic testing and ongoing monitoring, can be prohibitive for many patients, especially in low-income regions. This restricts access to the drug for some patient populations, limiting its potential market. Moreover, the need for constant treatment and monitoring further contributes to the financial burden on healthcare systems, making cost a significant barrier to widespread adoption.
Expanding indications and personalized treatments
One of the key opportunities in the Rydapt market lies in its expanding range of indications. Beyond treating AML, Rydapt is now being used to manage aggressive systemic mastocytosis (ASM) and mast cell leukemia (MCL), offering patients new treatment options. As research continues into Rydapt’s use in other cancers and genetic conditions, its potential market applications could grow significantly. Additionally, the growing trend of personalized medicine and genetic testing presents an opportunity to target more patients who can benefit from Rydapt’s specific mechanisms of action.
Regulatory hurdles and market competition
A major challenge facing the Rydapt market is the stringent regulatory requirements for drug approvals. The need for extensive clinical trials and lengthy approval processes can delay the availability of new treatments, hindering market growth. Additionally, competition from other targeted therapies and generics may pressure the market, particularly as patents for Rydapt may eventually expire. Maintaining market share in a competitive landscape and ensuring continued approval in various regions remains a challenge for the companies involved in Rydapt production.
The Rydapt market is witnessing several key trends that are shaping its future. First, the growing shift towards personalized medicine, driven by advancements in genomics and biomarker discovery, is expected to continue boosting demand for Rydapt, as it is highly effective for genetically-targeted cancers. Second, AI and data analytics are being increasingly used to streamline drug discovery, improve patient stratification, and enhance the monitoring of treatment efficacy, making Rydapt therapies more effective. Third, the broadening of Rydapt’s indications, from AML to other hematologic disorders, is creating new opportunities for growth in both developed and emerging markets. Furthermore, there is a rising focus on patient support programs, which aim to reduce the financial and logistical barriers to treatment access. These trends indicate that the Rydapt market will continue to evolve, with advancements in technology and personalized care playing a key role in its expansion.
The global Rydapt market is segmented by type, application, and end-user insights, each playing a crucial role in its growth trajectory. The market offers different types of Rydapt formulations including oral tablets, injectable solutions, and oral suspensions. Each formulation type caters to distinct patient needs and preferences, enhancing the accessibility and convenience of treatment. In terms of applications, Rydapt is used primarily for treating hematologic diseases like Acute Myeloid Leukemia (AML), Systemic Mastocytosis (SM), Chronic Eosinophilic Leukemia (CEL), and Hypereosinophilic Syndrome (HES). Each application has unique market dynamics based on disease prevalence and treatment efficacy. End-users such as hospitals, oncology clinics, and pharmaceutical companies drive the demand for Rydapt based on their roles in treatment administration, clinical development, and market distribution.
North America accounted for the largest market share at 40% in 2024, however, the Asia-Pacific region is expected to register the fastest growth, expanding at a CAGR of 23% between 2025 and 2032.
North America, Europe, and Asia-Pacific are the leading regions driving the growth of the Rydapt market. North America continues to hold the largest share, thanks to the high prevalence of blood disorders like Acute Myeloid Leukemia (AML) and Systemic Mastocytosis (SM). Europe follows closely, with strong healthcare infrastructure and an aging population fueling demand. Meanwhile, the Asia-Pacific region is set to experience the fastest growth, driven by increasing healthcare access and the rising incidence of hematologic diseases. The Middle East and Africa, though at a smaller scale, are also showing gradual expansion in the Rydapt market due to improvements in healthcare and drug accessibility.
A Leader in Advanced Cancer Therapies and Drug Adoption
North America leads the global Rydapt market, primarily driven by the demand for AML and Systemic Mastocytosis (SM) treatments. The United States remains the largest market due to a robust healthcare system, high treatment adoption, and the large patient population requiring therapies like Rydapt. In 2024, the U.S. accounted for more than 60% of the market share in North America. The increasing number of cancer patients, coupled with Rydapt's approval for multiple indications, further bolsters its usage. The strong research and clinical development environment in North America supports continued market growth, with both hospitals and oncology clinics driving demand.
Strong Healthcare Infrastructure Driving Cancer Treatment Demand
Europe is the second-largest market for Rydapt, with countries like Germany, France, and the UK contributing significantly to market growth. The growing aging population in Europe, particularly in Germany, is one of the key factors driving demand for treatments such as Rydapt. With high healthcare spending, European nations are investing heavily in advanced cancer therapies. Additionally, the approval of Rydapt for various hematological disorders has created new opportunities in the treatment of AML, SM, and other blood cancers. The European market is witnessing a steady increase in the number of Rydapt prescriptions, supported by healthcare providers' awareness and adoption of novel cancer treatments.
Rapid Healthcare Growth Fuels Rising Demand for Rydapt
The Asia-Pacific region is poised to experience the fastest growth in the Rydapt market, driven by rising healthcare access, an increasing incidence of hematologic diseases, and growing awareness of cancer treatments. Countries such as China, Japan, and India are seeing rapid improvements in healthcare infrastructure, making treatments like Rydapt more accessible to a broader population. The Asia-Pacific market is projected to grow rapidly due to the increasing prevalence of blood disorders and the region's expanding oncology treatment capabilities. As healthcare systems in this region continue to evolve, the demand for Rydapt is expected to increase significantly.
Emerging Markets Paving the Way for Advanced Treatments
The Middle East and Africa (MEA) are emerging markets for Rydapt, showing potential for growth as healthcare systems improve and access to modern therapies increases. While the MEA market currently represents a smaller share of the global Rydapt market, the region is witnessing an increasing prevalence of hematologic cancers, prompting greater demand for targeted therapies like Rydapt. Countries such as Saudi Arabia, the UAE, and South Africa are making strides in enhancing healthcare infrastructure, which, in turn, drives the availability and adoption of treatments like Rydapt. Additionally, the rising awareness of blood cancers is contributing to a gradual increase in market adoption.
The Rydapt market is highly competitive, with a few major pharmaceutical companies dominating the market space. Novartis, the manufacturer of Rydapt, continues to lead the market with its established presence in both North America and Europe. Other pharmaceutical companies are also exploring the treatment potential of Rydapt, particularly in the context of hematological diseases like AML and Systemic Mastocytosis. The increasing number of clinical trials and research activities is pushing competition, with companies focusing on improving drug formulations, patient outcomes, and treatment efficiencies. In this competitive landscape, Novartis is actively investing in expanding Rydapt's indications and treatment reach, securing its leadership position in the market.
Novartis
Bristol Myers Squibb
Pfizer
Roche
AbbVie
Merck & Co.
Sanofi
The Rydapt market is benefiting from technological advancements that improve the precision of treatments and enhance patient outcomes. One significant development is the integration of next-generation sequencing (NGS) technologies, which are increasingly used to detect FLT3 mutations. These mutations are crucial in identifying patients who would benefit from Rydapt therapy, enabling more targeted and effective treatments. The growing use of NGS technology in clinical settings has enhanced diagnostic capabilities, allowing for earlier and more accurate identification of the appropriate patient population.
Furthermore, advancements in drug delivery systems, such as extended-release formulations, are being explored to improve patient compliance. By reducing the frequency of dosing, these new formulations can lead to better patient adherence to treatment regimens. Another promising technology is the development of companion diagnostics, which ensures that Rydapt is prescribed to the right patients based on their genetic profiles. These technological innovations are streamlining treatment procedures, increasing the efficacy of Rydapt, and supporting its growth in the market. Overall, these advancements in technology are paving the way for more personalized and efficient treatment options, driving the demand for Rydapt and transforming the landscape of blood disorder therapies.
• In March 2024, Novartis, the manufacturer of Rydapt, announced a collaboration with several biotech companies to explore new indications for Rydapt in treating chronic eosinophilic leukemia (CEL) and systemic mastocytosis (SM). The collaboration aims to increase the therapeutic use of Rydapt in hematologic cancers.
• In January 2024, the U.S. FDA approved an expanded indication for Rydapt, allowing its use in combination therapy for treatment-resistant Acute Myeloid Leukemia (AML) patients, marking a significant milestone in AML treatment options.
• In December 2023, Novartis revealed that the European Medicines Agency (EMA) had granted Rydapt orphan drug designation for the treatment of hypereosinophilic syndrome (HES), a rare blood disorder, signaling increased opportunities for market expansion in the EU.
• In October 2023, a clinical trial in Canada showed positive results for Rydapt as part of a combination regimen for the treatment of AML with high-risk FLT3 mutations. This development further solidifies its position in advanced leukemia therapies.
• In August 2023, the American Society of Hematology (ASH) recognized Rydapt in their annual meeting for its groundbreaking advancements in treating systemic mastocytosis (SM) and AML, highlighting its increasing adoption in oncology clinics worldwide.
The Rydapt market report provides a comprehensive analysis of key market drivers, trends, and challenges, focusing on the development of new drug indications and advanced delivery mechanisms. The report highlights the role of technological advancements, such as next-generation sequencing and companion diagnostics, in expanding the market for Rydapt. It delves into the increasing prevalence of blood disorders, such as Acute Myeloid Leukemia (AML) and Systemic Mastocytosis (SM), which are driving the demand for targeted therapies. With a focus on geographic regions, the report also explores the growing market potential in North America, Europe, and Asia-Pacific, where healthcare infrastructure improvements are expected to support the adoption of Rydapt. It provides an in-depth look at the competitive landscape, detailing the key players such as Novartis and their ongoing innovations in the blood cancer treatment space. Furthermore, the report outlines the challenges and opportunities for market expansion, considering factors like the high cost of treatment and the increasing demand for personalized medicine. This report offers a detailed examination of the Rydapt market, providing valuable insights for stakeholders looking to capitalize on emerging opportunities in the blood disorder treatment sector.
| Report Attribute/Metric | Report Details |
|---|---|
|
Market Revenue in 2024 |
USD 660 Million |
|
Market Revenue in 2032 |
USD 3,113.81 Million |
|
CAGR (2025 - 2032) |
21.4% |
|
Base Year |
2024 |
|
Forecast Period |
2025 - 2032 |
|
Historic Period |
2020 - 2024 |
|
Segments Covered |
By Types
By Application
By End-User
|
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Region Covered |
North America, Europe, Asia-Pacific, South America, Middle East, Africa |
|
Key Players Analyzed |
Novartis, Bristol Myers Squibb, Pfizer, Roche, AbbVie, Merck & Co., Sanofi |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
