Reports
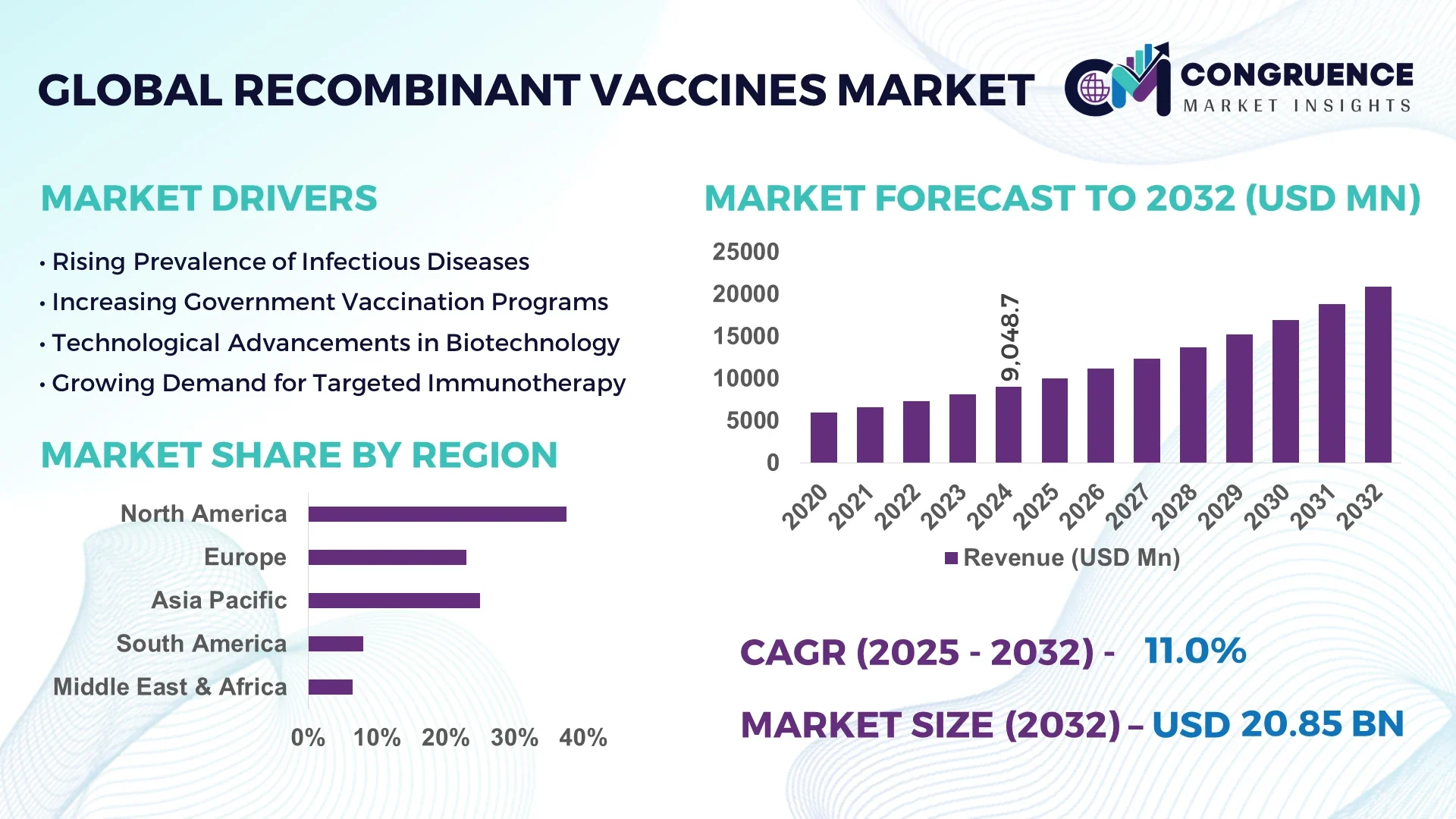
The Global Recombinant Vaccines Market was valued at USD 9,048.72 Million in 2024 and is anticipated to reach a value of USD 20,853.11 Million by 2032 expanding at a CAGR of 11.0% between 2025 and 2032.
To Learn More About This Report, Request A Free Sample Copy
The United States plays a pivotal role in the Recombinant Vaccines market, showcasing significant production capacity supported by advanced biotechnological infrastructure and substantial investment in research and development. The country’s vaccine manufacturing hubs are equipped with cutting-edge facilities enabling high-throughput production and rapid scalability. Furthermore, key applications across infectious disease prevention, oncology, and immunotherapy benefit from ongoing technological advancements, enhancing vaccine efficacy and safety profiles.
The Recombinant Vaccines Market is increasingly driven by multiple industry sectors, including pharmaceuticals, biotechnology, and healthcare providers, each contributing substantial market shares. Innovations such as vector-based and subunit vaccine platforms have revolutionized the market by improving immunogenicity and reducing adverse reactions. Regulatory frameworks worldwide emphasize stringent quality controls, accelerating approval timelines for recombinant vaccines that meet evolving safety standards. Environmental sustainability and cost-efficiency are gaining traction as manufacturers adopt green bioprocessing technologies. Regional consumption patterns reveal robust growth in emerging economies fueled by government immunization initiatives and rising public health awareness. Future market dynamics suggest a growing focus on personalized vaccines and mRNA recombinant technology, positioning the sector for continuous expansion and disruption.
Artificial intelligence (AI) is profoundly transforming the Recombinant Vaccines Market by streamlining research and development processes, enhancing operational efficiencies, and enabling more precise vaccine design. AI-powered algorithms facilitate the identification of optimal antigen targets by analyzing vast genomic and proteomic datasets, significantly reducing timeframes in early-stage vaccine discovery. This computational power allows manufacturers to predict immune responses and optimize recombinant constructs with higher accuracy, improving overall vaccine effectiveness.
In production environments, AI-driven automation and predictive maintenance tools optimize bioreactor performance and minimize downtime, directly increasing yield and reducing production costs. Moreover, AI-enabled data analytics support real-time monitoring of manufacturing processes, ensuring compliance with regulatory standards and quality assurance protocols. In clinical trial phases, AI applications assist in patient stratification and adaptive trial designs, accelerating trial completion and improving safety monitoring. These AI advancements not only improve the speed and efficiency of vaccine development but also facilitate the scalability of recombinant vaccine manufacturing to meet global demand. By integrating AI, the Recombinant Vaccines Market benefits from enhanced precision, reduced operational bottlenecks, and improved decision-making across the vaccine lifecycle.
“In 2024, a leading biotech firm implemented an AI-driven platform that reduced recombinant vaccine candidate screening time by 40%, enabling the evaluation of over 10,000 protein variants within weeks, significantly accelerating the development pipeline.”
The rising incidence of infectious diseases worldwide is a significant driver in the Recombinant Vaccines Market. Outbreaks of diseases such as hepatitis B, human papillomavirus (HPV), and novel viral infections have amplified the demand for safe and effective vaccines produced using recombinant DNA technology. Enhanced surveillance and vaccination campaigns globally have further boosted demand. For instance, recombinant vaccines offer improved safety profiles compared to traditional vaccines, making them preferred choices in immunization programs. Pharmaceutical companies are investing heavily in expanding recombinant vaccine pipelines to address unmet medical needs. This growing focus on disease prevention through innovative vaccine solutions sustains strong momentum in the market’s expansion.
One major restraint facing the Recombinant Vaccines Market is the high cost associated with production and development. The complexity of recombinant DNA technology requires advanced bioprocessing facilities, skilled workforce, and stringent quality control measures, all contributing to increased operational expenses. Manufacturing recombinant vaccines involves multiple stages, including gene cloning, expression, purification, and formulation, which can be resource-intensive. Additionally, prolonged clinical trial phases and regulatory compliance add further financial burdens. These elevated costs can limit accessibility and affordability, particularly in low- and middle-income regions, thereby constraining broader market penetration despite growing demand.
The expanding field of personalized vaccines presents a significant opportunity within the Recombinant Vaccines Market. Advances in genomics and immunology are enabling the development of vaccines tailored to individual genetic profiles and disease susceptibilities. Personalized recombinant vaccines offer potential breakthroughs in oncology and chronic disease management by targeting specific antigens unique to patients. This approach improves vaccine efficacy and reduces adverse effects, addressing unmet medical needs. Increasing collaborations between biotech firms and healthcare providers are fostering innovation in this space. As personalized medicine gains regulatory acceptance and market traction, recombinant vaccine manufacturers can capitalize on this emerging trend to diversify their product portfolios and access new therapeutic areas.
The Recombinant Vaccines Market faces significant challenges related to rigorous regulatory frameworks governing vaccine approval. Regulatory authorities impose stringent standards on safety, efficacy, and manufacturing consistency, which necessitate comprehensive clinical trials and extensive documentation. These processes often extend development timelines and require substantial investment. Variability in regulatory requirements across different regions complicates global market entry and distribution strategies. Additionally, concerns about potential adverse reactions and long-term safety necessitate ongoing post-market surveillance, increasing operational complexity. Navigating these regulatory hurdles demands significant expertise and resources from manufacturers, posing a barrier to rapid commercialization and market expansion.
• Expansion of mRNA-Based Recombinant Vaccines: The growing integration of mRNA technology within the Recombinant Vaccines market is transforming vaccine development approaches. mRNA-based recombinant vaccines enable faster design and production compared to traditional protein-based methods. In 2024 alone, multiple mRNA vaccine candidates advanced to late-stage clinical trials targeting infectious diseases beyond COVID-19, signaling broader acceptance and applicability. This trend is prompting manufacturers to invest in flexible manufacturing lines capable of handling mRNA vaccine production alongside conventional recombinant vaccines.
• Increased Use of Single-Use Bioreactors: Single-use bioreactor systems are gaining prominence in recombinant vaccine manufacturing due to their ability to reduce contamination risks and turnaround times. By 2025, adoption rates of disposable bioreactors in vaccine production facilities in Asia-Pacific and Europe have surged by over 30%, driven by the need for scalable and cost-effective manufacturing solutions. This shift allows manufacturers to respond quickly to fluctuating demand while minimizing cleaning and validation processes.
• Implementation of Advanced Purification Technologies: Advances in purification techniques such as membrane chromatography and continuous processing are becoming standard in the Recombinant Vaccines market. These technologies enhance yield and purity, critical factors for regulatory compliance and vaccine efficacy. Several leading vaccine manufacturers report a 20% improvement in downstream processing efficiency after integrating these methods, accelerating batch production without compromising quality.
• Growth of Digital Supply Chain Management: The Recombinant Vaccines market is witnessing increasing adoption of digital supply chain platforms incorporating AI and blockchain for enhanced transparency and traceability. These systems optimize inventory management, reduce waste, and improve logistics efficiency across global distribution networks. In North America and Europe, over 40% of vaccine manufacturers have implemented such technologies by 2024, facilitating faster response to supply disruptions and improving product delivery timelines.
The Recombinant Vaccines Market is segmented by product type, application, and end-user categories, each offering distinct insights into industry trends and demand drivers. Product segmentation includes protein subunit vaccines, vector-based vaccines, DNA vaccines, and others, reflecting diverse technological platforms. Application-wise, recombinant vaccines are utilized across infectious diseases, oncology, autoimmune disorders, and veterinary medicine, highlighting their versatile therapeutic scope. End-user segmentation covers hospitals, clinics, research institutes, and pharmaceutical companies, illustrating the varied stakeholders involved in vaccine deployment and development. Understanding these segments enables industry decision-makers to target growth areas effectively and align strategic initiatives with evolving market needs.
Protein subunit vaccines dominate the Recombinant Vaccines market due to their well-established safety profile and efficacy in preventing viral infections such as hepatitis B and HPV. Their widespread use in immunization programs worldwide supports their leading position, backed by extensive clinical validation and regulatory acceptance. Vector-based vaccines are the fastest-growing segment, driven by advances in viral vector technology and successful deployment in recent pandemic responses. Their ability to induce strong immune responses and versatility across different pathogens fuels this rapid growth. DNA vaccines, although still emerging, hold significant promise due to ease of design and manufacturing flexibility, particularly in personalized medicine and cancer immunotherapy. Other types, including conjugate and toxoid recombinant vaccines, occupy niche roles primarily in veterinary applications and specialized human vaccines, contributing to the market’s technological diversity.
Infectious diseases remain the leading application for recombinant vaccines, reflecting their critical role in global immunization efforts targeting hepatitis, influenza, and HPV infections. Government vaccination campaigns and public health initiatives continue to drive substantial demand in this segment. Oncology applications represent the fastest-growing area, propelled by advances in recombinant vaccine platforms that target tumor-specific antigens to stimulate immune-mediated cancer therapy. Research and clinical trial activities in cancer immunotherapy are expanding rapidly, boosting interest and investment. Autoimmune disorders and veterinary medicine also contribute to the application landscape, with recombinant vaccines offering novel treatment and preventive solutions. Veterinary applications, in particular, are gaining importance due to increasing livestock health management and zoonotic disease control efforts.
Hospitals are the primary end-users in the Recombinant Vaccines market, serving as key points for vaccine administration in both inpatient and outpatient settings. Their central role in mass immunization drives consistent demand, supported by healthcare infrastructure improvements in many regions. Research institutes are the fastest-growing end-user segment, fueled by rising investments in vaccine innovation and personalized medicine development. These institutes play a critical role in early-stage research, clinical trials, and collaborative projects with biotech firms. Pharmaceutical companies also significantly contribute to the market through large-scale production and distribution, while clinics, especially in emerging economies, are expanding access to recombinant vaccines through outpatient services. This diversified end-user base ensures broad market penetration and ongoing innovation.
North America accounted for the largest market share at 37.5% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 13.5% between 2025 and 2032.
To Learn More About This Report, Request A Free Sample Copy
North America continues to lead due to its advanced biotechnology infrastructure and robust pharmaceutical industry investment. The region benefits from extensive R&D facilities focused on recombinant vaccines for infectious diseases and cancer. Asia-Pacific's growing healthcare expenditure, expanding manufacturing capabilities, and increasing government initiatives to improve vaccine accessibility are key growth factors. Europe and South America also contribute significantly, supported by regulatory frameworks and increasing adoption of innovative vaccine technologies. Middle East & Africa is witnessing steady demand growth driven by emerging healthcare infrastructure and international trade partnerships.
Innovative Biotech Advancements Driving Market Leadership
North America holds approximately 38% of the global Recombinant Vaccines market volume, fueled by demand from pharmaceutical and biotechnology sectors focused on infectious disease prevention and oncology vaccines. Government initiatives promoting vaccine development and distribution, including streamlined regulatory pathways, have accelerated product approvals. Digital transformation in manufacturing, such as automation and AI-driven analytics, enhances operational efficiency. The presence of key industry players and cutting-edge research institutions further supports sustained innovation and market expansion. Regulatory agencies continuously update guidelines to ensure safety and efficacy, boosting confidence among stakeholders and end-users.
Strategic Collaborations and Sustainability Efforts Shaping Growth
Europe captures nearly 25% market share, with Germany, the UK, and France as primary contributors to recombinant vaccine demand. The region’s regulatory bodies enforce stringent quality controls, encouraging vaccine safety and sustainability initiatives aligned with environmental policies. European markets emphasize the adoption of advanced bioprocessing technologies, including continuous manufacturing and single-use systems, to reduce production time and environmental footprint. Public-private partnerships and increased funding for vaccine innovation are notable trends, supporting expansion in both human and veterinary recombinant vaccine sectors.
Emerging Innovation Hubs and Expanding Manufacturing Capacities
Asia-Pacific ranks second globally in recombinant vaccine consumption, accounting for approximately 22% of market volume in 2024. China, India, and Japan lead demand due to large population bases and expanding healthcare infrastructure. Manufacturing facilities in the region are adopting state-of-the-art technologies such as modular biomanufacturing plants to increase flexibility and output. Innovation hubs in urban centers facilitate research on next-generation recombinant vaccines targeting region-specific infectious diseases. Government policies promoting biotechnology exports and investments in cold-chain logistics are also driving market development.
Growing Infrastructure and Trade Incentives Accelerating Market Growth
South America represents about 8% of the recombinant vaccines market share, with Brazil and Argentina as dominant players. Regional infrastructure improvements, including new vaccine production sites and upgraded cold storage facilities, support increased manufacturing and distribution capabilities. Government incentives aimed at boosting local pharmaceutical industries, combined with trade agreements facilitating vaccine exports, are significant contributors to market progress. Additionally, rising demand for vaccines in veterinary applications aligns with the region’s strong agricultural sector.
Technological Modernization and Strategic Partnerships Driving Demand
The Middle East & Africa region accounts for roughly 7% of the recombinant vaccines market, with the UAE and South Africa leading growth efforts. The market benefits from increased investments in healthcare infrastructure modernization and enhanced regulatory frameworks facilitating vaccine approval processes. Trade partnerships with global biotech firms enable technology transfer and local production capacity building. Demand is notably rising within sectors such as oil & gas and construction, where workforce health and safety protocols include immunization programs, further boosting market uptake.
United States: Holds approximately 35% market share, driven by extensive production capacity and robust end-user demand in both human and veterinary vaccine sectors.
China: Accounts for around 20% market share, supported by significant government investment in biotech manufacturing infrastructure and growing domestic consumption.
The competitive environment in the Recombinant Vaccines market is marked by the presence of over 40 active global players, ranging from established pharmaceutical giants to innovative biotech startups. Market positioning is largely influenced by companies’ investment in research and development, strategic partnerships, and expanding production capacities. Leading firms are increasingly focusing on launching novel recombinant vaccine candidates targeting infectious diseases, cancer, and other therapeutic areas. Collaborations between biotech companies and academic institutions are common, accelerating innovation and reducing time-to-market for new products. Mergers and acquisitions also play a critical role, enabling companies to diversify their product portfolios and strengthen market reach. Technological advancements such as gene editing, synthetic biology, and advanced bioprocessing are driving competitive differentiation. Furthermore, firms are investing in digital technologies like AI and automation to enhance manufacturing efficiency and supply chain resilience. Overall, the market competition is dynamic, with players prioritizing innovation, regulatory compliance, and geographic expansion to secure and enhance their market positions.
Pfizer Inc.
Novavax, Inc.
Sanofi S.A.
GlaxoSmithKline plc
Merck & Co., Inc.
Bharat Biotech International Ltd.
CSL Limited
Dynavax Technologies Corporation
Bavarian Nordic A/S
Serum Institute of India Pvt. Ltd.
The Recombinant Vaccines market is significantly influenced by cutting-edge biotechnologies that enhance vaccine development, production, and delivery efficiency. Advanced genetic engineering techniques, including CRISPR and gene editing, enable precise antigen design to improve immune response specificity. Additionally, next-generation sequencing (NGS) platforms accelerate pathogen genome analysis, facilitating rapid vaccine candidate identification and optimization. Bioprocessing technologies such as single-use bioreactors and continuous manufacturing systems are increasingly adopted to reduce contamination risks and shorten production cycles while maintaining high-quality standards.
Moreover, innovations in adjuvant formulation and nanoparticle delivery systems improve vaccine stability and efficacy, expanding the scope of recombinant vaccines to address complex diseases. Digital transformation initiatives, including AI-driven predictive modeling and machine learning algorithms, optimize vaccine design and clinical trial outcomes by identifying potential safety and efficacy concerns early. Automation and robotics in vaccine manufacturing streamline operations, reduce human error, and increase batch consistency. Cold chain innovations, like advanced cryopreservation and temperature monitoring technologies, are critical for maintaining recombinant vaccine integrity during storage and distribution, especially in emerging markets with challenging logistics. Collectively, these technology advancements position the Recombinant Vaccines market for sustained innovation, enabling companies to meet growing global demand and regulatory expectations.
In March 2024, Moderna announced the initiation of a phase 3 clinical trial for its new recombinant RSV vaccine, targeting older adults and infants, with improved immunogenicity demonstrated in preliminary results.
In November 2023, Pfizer expanded its manufacturing capacity for recombinant COVID-19 vaccines by opening a new biologics production facility in Europe, aimed at increasing annual output by 20 million doses.
In January 2024, Novavax received regulatory approval for its recombinant nanoparticle influenza vaccine in multiple Asian markets, marking a significant expansion in its global footprint.
In September 2023, Sanofi launched a next-generation recombinant dengue vaccine, incorporating novel adjuvant technology to enhance protective efficacy, targeting endemic regions in Latin America and Southeast Asia.
The Recombinant Vaccines Market Report offers an extensive analysis of key market segments, encompassing product types such as subunit vaccines, vector-based vaccines, and DNA vaccines. The report examines applications across diverse disease areas including infectious diseases, oncology, and autoimmune disorders, reflecting the broad therapeutic relevance of recombinant vaccine technologies. It also covers the pharmaceutical, biotechnology, and healthcare sectors as primary end-users, providing insights into their evolving demand patterns and operational priorities. Geographically, the report provides a comprehensive overview of regional markets including North America, Europe, Asia-Pacific, South America, and the Middle East & Africa, highlighting distinctive market dynamics, regulatory frameworks, and infrastructure developments in each region. It details the technological landscape by focusing on innovations like gene editing, nanoparticle delivery systems, and digital manufacturing platforms that drive market advancement.
Additionally, the scope includes analysis of emerging trends such as personalized recombinant vaccines and mRNA-based technologies, which are gaining traction in precision medicine and pandemic preparedness. The report also identifies niche segments such as veterinary recombinant vaccines, underscoring potential growth avenues. By synthesizing market size, segmentation, technological trends, and competitive landscapes, this report serves as a critical resource for stakeholders aiming to make informed strategic decisions in the recombinant vaccines industry.
| Report Attribute/Metric | Report Details |
|---|---|
|
Market Revenue in 2024 |
USD 9048.72 Million |
|
Market Revenue in 2032 |
USD 20853.11 Million |
|
CAGR (2025 - 2032) |
11% |
|
Base Year |
2024 |
|
Forecast Period |
2025 - 2032 |
|
Historic Period |
2020 - 2024 |
|
Segments Covered |
By Types
By Application
By End-User
|
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Region Covered |
North America, Europe, Asia-Pacific, South America, Middle East, Africa |
|
Key Players Analyzed |
Pfizer Inc., Novavax, Inc., Sanofi S.A., GlaxoSmithKline plc, Merck & Co., Inc., Bharat Biotech International Ltd., CSL Limited, Dynavax Technologies Corporation, Bavarian Nordic A/S, Serum Institute of India Pvt. Ltd. |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
