Reports
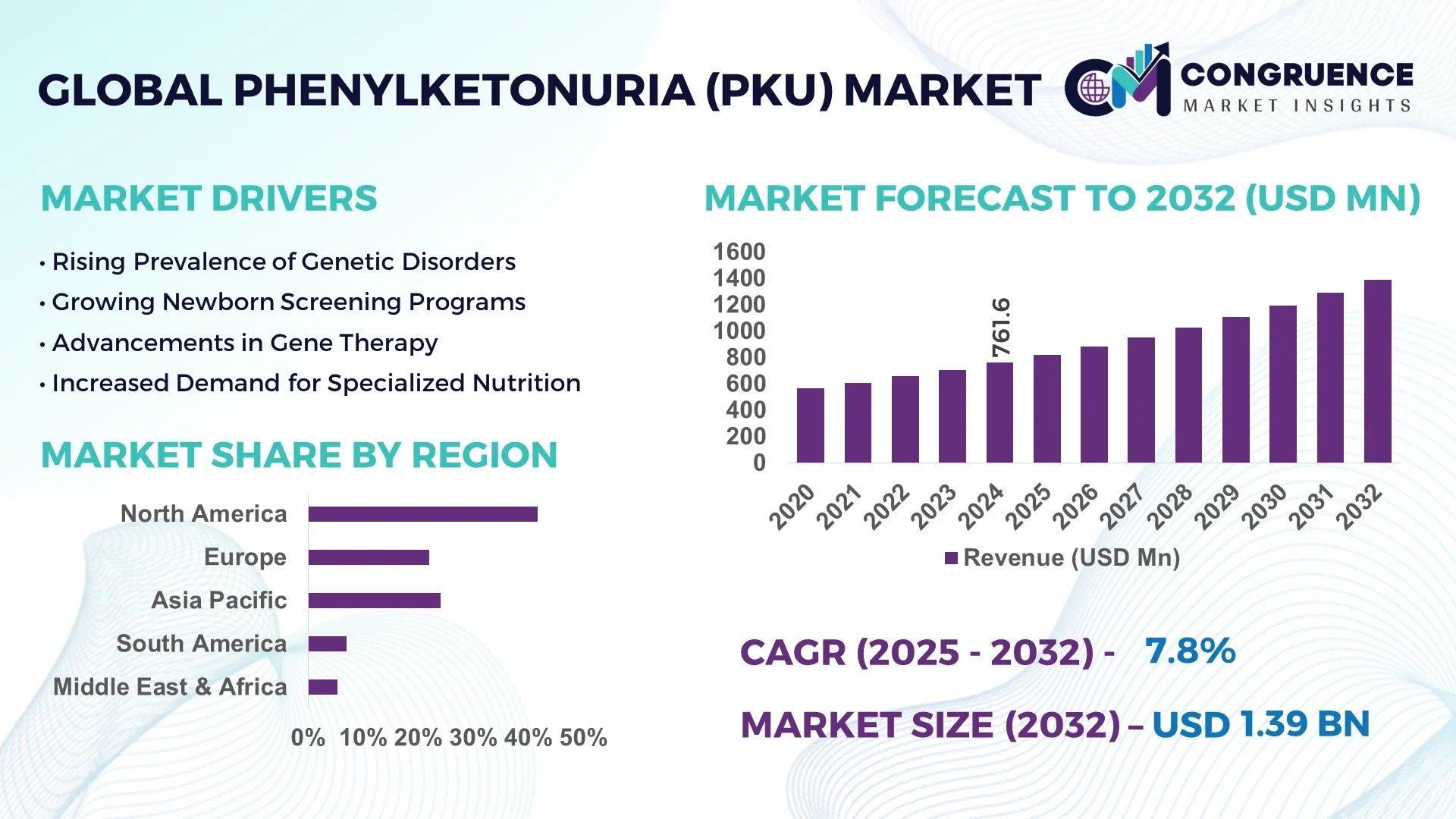
The Global Phenylketonuria (PKU) Market was valued at USD 761.6 Million in 2024 and is anticipated to reach a value of USD 1,388.9 Million by 2032 expanding at a CAGR of 7.8% between 2025 and 2032.
To Learn More About This Report, Request A Free Sample Copy
North America dominates the global PKU market, with the United States being the major contributor due to the presence of advanced healthcare infrastructure, high awareness levels, and favorable reimbursement policies. Moreover, early newborn screening programs are extensively implemented, aiding in the timely diagnosis and treatment of PKU.
The PKU market is driven by ongoing innovation in treatment modalities, including enzyme substitution therapies and gene therapy solutions. Additionally, the rise in dietary management approaches, such as specialized low-protein foods and medical nutrition products, continues to enhance patient quality of life. The prevalence of PKU is about 1 in 10,000 to 15,000 newborns in the U.S., leading to a significant patient pool requiring lifelong care. As a result, government initiatives and collaborations with biotech firms have intensified, encouraging the development of sustainable and long-term therapeutic options.
Artificial Intelligence (AI) is making a profound impact on the Phenylketonuria (PKU) market by revolutionizing diagnostic processes, patient monitoring, and personalized treatment strategies. AI-driven platforms are increasingly used to optimize dietary recommendations for PKU patients based on real-time metabolic data. This helps in maintaining phenylalanine levels within a safe range, reducing the risk of neurological damage.
Machine learning models are now used in conjunction with wearable devices and electronic health records to track patient adherence to low-protein diets. These models can predict metabolic fluctuations and flag potential health risks before symptoms become severe. AI also enhances the screening process for newborns through advanced image recognition and pattern detection in tandem mass spectrometry outputs, ensuring earlier and more accurate diagnoses.
Moreover, AI-powered data analysis is aiding clinical trials by identifying the most responsive patient subgroups for emerging therapies. This shortens development timelines and improves the efficiency of resource allocation. AI's predictive capabilities further help healthcare providers to anticipate long-term patient outcomes and adjust treatment plans dynamically.
"In April 2024, a U.S.-based biotechnology company integrated AI algorithms with next-generation sequencing to improve the diagnostic accuracy of PKU, reducing false positives in newborn screenings by 35%. This innovation has also shortened the average time to diagnosis by 48 hours, significantly improving early intervention outcomes."
The advancement and increasing adoption of enzyme replacement therapies (ERT) and gene therapies are significantly driving the PKU market. These therapies offer more targeted and effective treatment options than traditional methods. Drugs like pegvaliase (Palynziq) have demonstrated efficacy in reducing phenylalanine levels in adults with uncontrolled PKU. Gene therapy approaches under development aim to provide a one-time cure, reducing dependency on lifelong dietary restrictions. This medical shift is encouraging investments in biopharmaceutical R&D, contributing to the market’s expansion.
One of the primary restraints of the PKU market is the high cost of therapies, especially innovative treatments such as enzyme substitution and gene therapy. These therapies often require long-term monitoring and personalized adjustments, which significantly add to the total treatment cost. Additionally, access to these treatments is limited in low- and middle-income countries due to the lack of advanced healthcare infrastructure and inadequate reimbursement frameworks. This results in a treatment gap, where a significant portion of the patient population remains underserved.
The convergence of digital health and personalized nutrition offers significant opportunities in the PKU market. Mobile apps and AI-integrated platforms are being developed to provide customized meal plans, monitor phenylalanine levels, and offer telemedicine consultations. These innovations are particularly beneficial for pediatric patients and individuals in remote areas. The integration of such tools is increasing patient adherence to dietary management and reducing complications, thereby improving overall treatment outcomes and market reach.
A major challenge in the global PKU market is the limited awareness and late diagnosis, particularly in developing countries. Newborn screening programs are either absent or underutilized in several regions, resulting in delayed identification of PKU cases. This delay can lead to severe neurological impairments and long-term disabilities. Additionally, cultural beliefs, lack of trained healthcare professionals, and infrastructural constraints further exacerbate the issue, hindering early intervention and affecting the efficacy of available treatments.
Increasing Adoption of Advanced Therapies: The market is witnessing a shift toward advanced therapies like gene therapy and enzyme replacement therapy. These options provide a more effective and sometimes permanent solution compared to conventional dietary management. Clinical trials are focusing on long-acting treatments to reduce the burden of daily monitoring and improve compliance.
Rise in Specialized Medical Nutrition Products: There is an increasing demand for specialized low-protein foods and medical nutrition products tailored for PKU patients. These are formulated to be palatable, nutritionally adequate, and aligned with patient needs, particularly in pediatric care. Companies are investing in taste-enhancing technologies and convenient formats such as ready-to-eat meals and drinks.
Digital Health and Monitoring Platforms: The adoption of mobile apps and AI-based monitoring systems is transforming disease management. These tools provide real-time tracking of dietary intake and phenylalanine levels, enhancing adherence and clinical outcomes. They are particularly useful in managing pediatric cases and improving communication between patients and healthcare providers.
Expansion of Newborn Screening Programs: Global expansion of mandatory newborn screening is a major trend influencing the PKU market. Countries are increasingly adopting universal screening policies, leading to earlier diagnoses and timely initiation of dietary or pharmaceutical interventions. This trend supports improved long-term outcomes and reduces the societal burden of untreated PKU.
The Phenylketonuria (PKU) market is segmented based on type, application, and end-user, each playing a critical role in shaping market dynamics. This segmentation allows for a deeper understanding of targeted therapies and demand patterns. By dissecting the market across these segments, stakeholders can align product development strategies with patient needs and healthcare trends. Specific treatment types such as medications and dietary supplements are witnessing varied growth rates. Applications focus on diagnosis and treatment monitoring, while end-users range from hospitals to homecare settings, each with distinct adoption trends.
The PKU market by type includes Classic PKU, Mild PKU, and Benign Hyperphenylalaninemia. Classic PKU holds the largest market share due to its severe phenotype requiring strict and lifelong management. It accounts for more than 60% of the diagnosed cases globally. The demand for continuous enzyme therapy and nutritional supplements in Classic PKU is significantly high.
Mild PKU is the fastest-growing segment, driven by improved diagnostic techniques and broader screening that identifies less severe forms of the disease earlier. While the therapeutic demands are less intense, the segment is benefiting from increasing focus on personalized nutrition and moderate dietary interventions. Benign Hyperphenylalaninemia, although least severe, remains stable in its demand for monitoring and occasional dietary support, contributing moderately to market growth. Innovations in genetic profiling are helping refine these classifications, supporting better treatment alignment for each phenotype.
The application segments in the PKU market include Diagnosis, Treatment, and Monitoring. Treatment remains the dominant application segment, propelled by a growing number of patients requiring continuous medical and dietary intervention. Therapeutic advancements like enzyme substitution and potential gene therapy have expanded treatment options significantly.
Monitoring is emerging as the fastest-growing segment due to the rise of digital health technologies and AI-powered platforms. These solutions provide real-time phenylalanine tracking and dietary adherence monitoring, enabling proactive healthcare interventions. As patients and caregivers increasingly adopt these tools, the segment is expected to witness substantial growth.
Diagnosis continues to be critical, especially in newborn screening programs that form the foundation of early PKU management. The introduction of AI-enhanced diagnostics and mass spectrometry has improved diagnostic accuracy and reduced the time to intervention. Though its market share is stable, ongoing technology integration is ensuring its relevance and continued importance in patient care.
End-users of PKU treatments and services include Hospitals, Specialty Clinics, and Homecare Settings. Hospitals represent the leading end-user segment, owing to the availability of comprehensive diagnostic tools, multidisciplinary teams, and capacity for emergency care. The role of hospitals is especially crucial during the initial diagnosis and treatment initiation phases.
Homecare Settings are emerging as the fastest-growing end-user segment. The shift toward home-based disease management, driven by digital health platforms and user-friendly nutritional solutions, supports convenience and long-term adherence to treatment protocols. Patients increasingly rely on telemedicine consultations and app-based dietary support, enhancing outcomes in the home environment.
Specialty Clinics also contribute significantly, especially in urban centers with dedicated metabolic disorder facilities. These clinics often provide focused care, including genetic counseling and tailored dietary plans. As awareness rises and more clinics adopt advanced tools for PKU management, their role in delivering patient-centric care is expected to grow steadily.
North America accounted for the largest market share at 41.7% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 9.4% between 2025 and 2032.
To Learn More About This Report, Request A Free Sample Copy
North America's dominance is attributed to its robust newborn screening programs, high healthcare spending, and early adoption of innovative therapies. Meanwhile, Asia-Pacific is witnessing significant growth due to increasing awareness, rising birth rates, and expanding healthcare infrastructure in countries like China and India. Additionally, government efforts in these regions to introduce or improve early screening and diagnosis of rare disorders are catalyzing market expansion.
In North America, particularly the United States and Canada, PKU diagnosis and treatment are supported by established newborn screening mandates and insurance coverage for essential dietary treatments. Over 98% of newborns are screened for PKU at birth, ensuring timely diagnosis. The region has also witnessed the rapid approval of advanced therapies, such as pegvaliase for adult patients. Strategic collaborations between pharmaceutical companies and research institutions are accelerating product development and market penetration. Further, the market is benefiting from a supportive regulatory environment that facilitates fast-track approvals for rare disease treatments.
Europe holds a significant share of the PKU market, with countries like Germany, the UK, and France leading in screening coverage and treatment adoption. The region is characterized by strong public health systems that subsidize medical foods and treatment costs. Innovative nutritional therapies tailored to specific PKU types are being widely adopted, especially in pediatric care. Europe also boasts a growing network of specialized metabolic disorder clinics that offer comprehensive care, including psychological support, genetic counseling, and personalized treatment planning.
The Asia-Pacific region is emerging as a promising market due to increasing public health investments and awareness campaigns. Countries like China, Japan, and India are improving their newborn screening coverage, which is crucial for timely PKU management. Japan already mandates nationwide screening, while China is expanding regional pilot programs. Local production of low-protein foods and imported dietary solutions are becoming more accessible. Additionally, technological integration such as AI-supported diagnostics is gaining traction, especially in urban medical centers, contributing to market acceleration.
In South America, Brazil and Argentina are taking the lead in PKU awareness and diagnosis efforts. Brazil's public health system offers free newborn screening for metabolic disorders, including PKU. However, the region faces challenges such as limited availability of medical foods and slow approval processes for advanced therapies. Non-governmental organizations play a key role in supporting families through awareness programs and treatment access. Despite economic limitations, policy reforms and public health advocacy are steadily improving PKU care infrastructure.
The Middle East & Africa region currently lags in terms of market share, but nations like the UAE and South Africa are showing improvement. Newborn screening programs are expanding, though inconsistently across countries. Increasing investment in rare disease diagnostics and collaborations with international healthcare providers are supporting gradual growth. Challenges remain in terms of awareness, specialist availability, and access to medical foods. However, governments are initiating partnerships with NGOs and global health agencies to bridge these gaps and promote early diagnosis and dietary support.
Top Two Countries by Market Share in 2024
United States: USD 270.1 Million in 2024 – Due to early screening, advanced treatment options, and high per capita healthcare spending.
Germany: USD 84.5 Million in 2024 – Supported by comprehensive healthcare coverage and widespread adoption of nutritional therapies.
The Phenylketonuria (PKU) market is characterized by intense competition among key players focusing on innovative therapies and comprehensive treatment solutions. Companies are actively investing in research and development to introduce advanced enzyme replacement therapies, gene therapies, and specialized dietary products. Strategic collaborations, mergers, and acquisitions are common strategies employed to enhance product portfolios and expand market reach. For instance, in August 2024, Otsuka Holdings Co., Ltd. agreed to acquire Jnana Therapeutics Inc., a biotechnology company known for its PKU drug, in a deal worth up to USD 800 million, aiming to improve Otsuka's rare metabolic disease portfolio and accelerate Jnana's drug development. Additionally, in March 2024, PTC Therapeutics, Inc. submitted the sepiapterin Marketing Authorization Application (MAA) to the European Medicines Agency, targeting both juvenile and adult PKU patients across all disease subgroups. These developments indicate a dynamic market landscape with companies striving to address unmet medical needs and introduce personalized treatment options.
BioMarin Pharmaceutical Inc.
PTC Therapeutics, Inc.
Homology Medicines, Inc.
Synlogic, Inc.
Codexis, Inc.
Erytech Pharma SA
Relief Therapeutics Holding SA
Nestlé Health Science
Cambrooke Therapeutics
Vitaflo International
Abbott Laboratories
APR Applied Pharma Research S.A.
Agios Pharmaceuticals Inc.
Rubius Therapeutics, Inc.
Dimension Therapeutics, Inc.
Orchard Therapeutics
Aeglea BioTherapeutics
Medunik USA
SOM Innovation Biotech SL
American Gene Technologies International Inc.
Technological advancements are playing a pivotal role in transforming the PKU market, offering innovative solutions for diagnosis, treatment, and patient management. The integration of next-generation sequencing (NGS) and tandem mass spectrometry has significantly enhanced the accuracy and efficiency of PKU testing, facilitating early detection and personalized treatment plans. Artificial intelligence (AI) and machine learning algorithms are being utilized to optimize dietary recommendations and monitor phenylalanine levels in real-time, improving patient adherence and outcomes. Moreover, the development of enzyme replacement therapies, such as Palynziq (pegvaliase), has provided effective options for patients unresponsive to traditional treatments. Gene therapy approaches are also under investigation, aiming to offer long-term solutions by correcting the underlying genetic defects. Additionally, the emergence of synthetic biotic medicines, like Synlogic's SYNB1934, represents a novel approach to PKU treatment, utilizing engineered bacteria to metabolize phenylalanine. These technological innovations are collectively contributing to a more comprehensive and effective management of PKU, addressing the diverse needs of patients and healthcare providers.
In August 2024, Otsuka Holdings Co., Ltd. agreed to acquire Jnana Therapeutics Inc., a biotechnology company known for its PKU drug, in a deal worth up to USD 800 million. This acquisition aims to enhance Otsuka's rare metabolic disease portfolio and expedite Jnana's drug development efforts.
In March 2024, PTC Therapeutics, Inc. submitted the sepiapterin Marketing Authorization Application (MAA) to the European Medicines Agency. This submission targets both juvenile and adult PKU patients across all disease subgroups, potentially expanding therapy options for these patients.
In July 2024, Homology Medicines, Inc. launched Palynziq, a new enzyme replacement therapy (ERT) for phenylketonuria (PKU). This treatment is designed to lower blood phenylalanine levels in adults with PKU who struggle with current therapies, marking a significant advancement in PKU management.
In January 2023, Jnana Therapeutics announced that it received FDA clearance for the Investigational New Drug (IND) application for its drug JNT-517, developed to treat PKU. This clearance allows the company to proceed with clinical trials, potentially introducing a new treatment option for PKU patients.
The Phenylketonuria (PKU) Market Report provides a comprehensive analysis of the current market landscape, encompassing various aspects such as market size, growth drivers, challenges, and emerging trends. The report delves into segmentation by PKU type, treatment type, age group, route of administration, and end-user, offering detailed insights into each category. It highlights the increasing prevalence of PKU, advancements in diagnostic technologies, and the development of innovative therapies as key factors propelling market growth. The report also examines the competitive landscape, profiling major players and analyzing their strategies to gain a competitive edge. Furthermore, it explores regional market dynamics, identifying North America as the largest market due to established newborn screening programs and advanced healthcare infrastructure, while Asia-Pacific is anticipated to witness the fastest growth owing to rising awareness and healthcare investments. The report serves as a valuable resource for stakeholders, providing actionable insights to inform strategic decision-making and capitalize on emerging opportunities in the PKU market.
| Report Attribute / Metric | Report Details |
|---|---|
| Market Name | Global Phenylketonuria (PKU) Market |
| Market Revenue (2024) | USD 761.6 Million |
| Market Revenue (2032) | USD 1,388.9 Million |
| CAGR (2025–2032) | 7.8% |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User
|
| Key Report Deliverables | Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape, Technological Insights, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | BioMarin Pharmaceutical Inc., PTC Therapeutics, Inc., Homology Medicines, Inc., Synlogic, Inc., Codexis, Inc., Erytech Pharma SA, Relief Therapeutics Holding SA, Nestlé Health Science, Cambrooke Therapeutics, Vitaflo International, Abbott Laboratories, APR Applied Pharma Research S.A., Agios Pharmaceuticals Inc., Rubius Therapeutics, Inc., Dimension Therapeutics, Inc., Orchard Therapeutics, Aeglea BioTherapeutics, Medunik USA, SOM Innovation Biotech SL, American Gene Technologies International Inc. |
| Customization & Pricing | Available on Request (10% Customization Free) |
