Reports
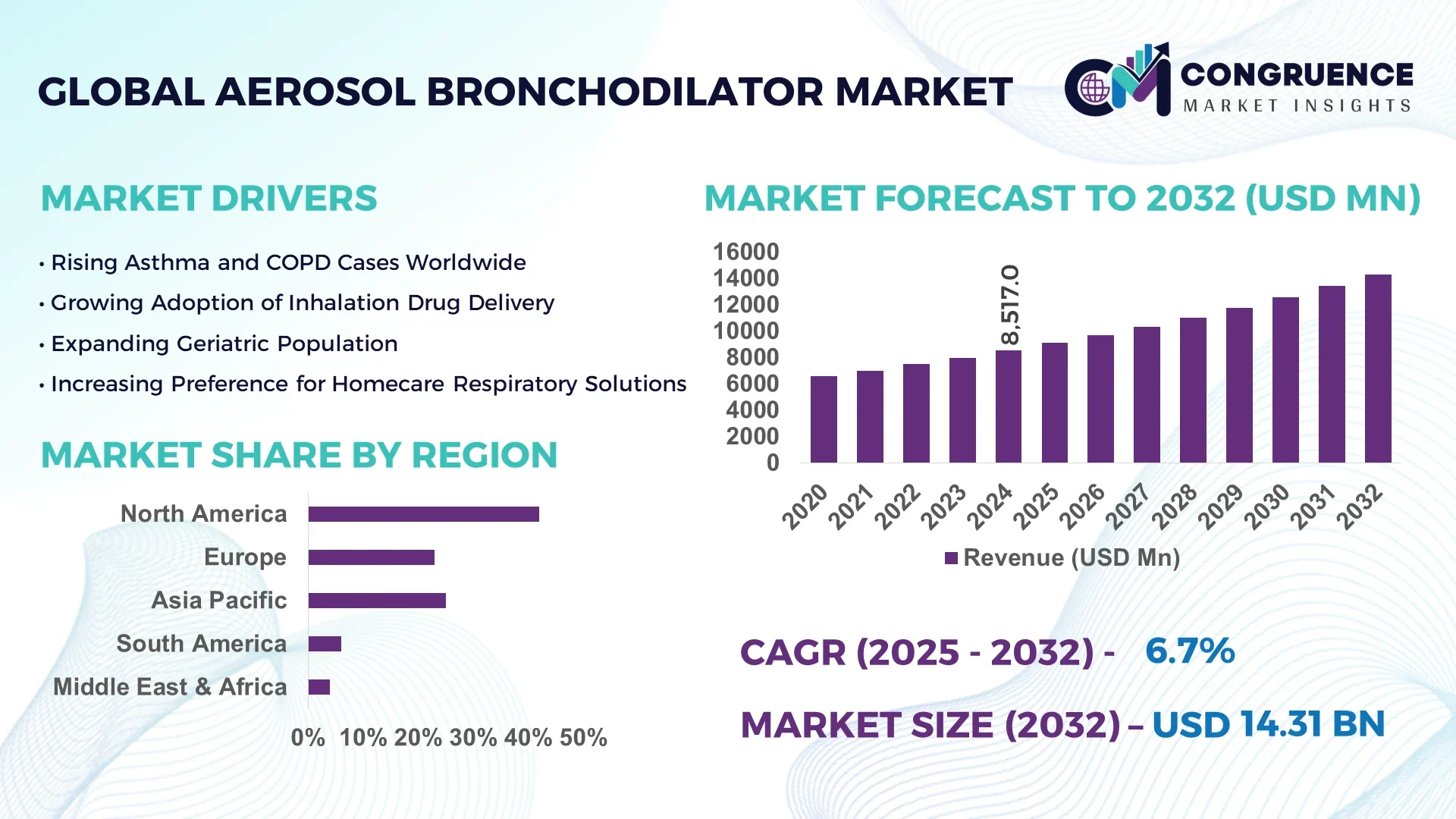
The Global Aerosol Bronchodilator Market was valued at USD 8,517 Million in 2024 and is anticipated to reach a value of USD 14,308.76 Million by 2032 expanding at a CAGR of 6.7% between 2025 and 2032.
The United States leads the global Aerosol Bronchodilator market with significant production capacity, advanced manufacturing facilities, high R&D investment in inhalation therapies, and a strong base of industry applications across hospitals and homecare sectors, supported by rapid adoption of advanced drug-delivery technologies.
The Aerosol Bronchodilator Market serves critical applications within respiratory care, notably in managing asthma, COPD, and bronchospasm treatment, with significant consumption patterns across North America, Europe, and Asia-Pacific. The market has seen consistent growth driven by rising respiratory disease prevalence, urban pollution, and an aging global population requiring chronic respiratory care. Technological advancements include the integration of digital dose counters and smart inhalers with Bluetooth-enabled monitoring to improve patient adherence and medication efficiency. Regulatory advancements in inhalation product approvals and environmental guidelines promoting eco-friendly propellants are reshaping production practices. Industry sectors such as hospital pharmacies, retail pharmacies, and online distribution channels are contributing actively to market growth, while increasing healthcare infrastructure investments and expanded reimbursement policies in emerging economies are enhancing market accessibility. The future outlook for the Aerosol Bronchodilator Market indicates a strong trajectory supported by the integration of AI and IoT in inhaler devices, improved drug formulations, and personalized medicine approaches in respiratory care.
Artificial Intelligence is transforming the Aerosol Bronchodilator Market by enhancing predictive analytics, improving patient adherence, and streamlining drug delivery mechanisms across the respiratory care ecosystem. AI-powered algorithms are enabling the development of smart inhalers that monitor inhalation patterns, medication usage, and patient respiratory rates in real time, helping clinicians adjust treatment plans precisely. These AI-driven inhalers in the Aerosol Bronchodilator Market provide alerts to patients, reducing missed doses and improving therapeutic outcomes, which is particularly crucial in asthma and COPD management.
AI integration within the Aerosol Bronchodilator Market has led to the automation of inhaler quality control, reducing manual errors in dose calibration and ensuring consistent delivery efficiency. Manufacturers are utilizing machine learning models to analyze patient feedback and device performance data, enabling the design of advanced inhalers with reduced aerosol wastage and optimized dose distribution. AI-powered platforms are being integrated into clinical trials, facilitating faster and more accurate data analysis, reducing the time needed for new product development within the Aerosol Bronchodilator Market. Additionally, AI applications are being leveraged in predictive maintenance of inhaler manufacturing equipment, reducing operational downtimes and enhancing production efficiency in high-demand regions. Healthcare providers are also using AI to stratify patients based on disease severity and inhalation technique efficiency, allowing personalized bronchodilator delivery strategies. These innovations are collectively positioning AI as a pivotal driver of operational transformation, advanced product development, and personalized respiratory care within the Aerosol Bronchodilator Market.
“In March 2025, a leading U.S.-based respiratory solutions company launched an AI-powered aerosol bronchodilator inhaler that monitors inhalation angles and respiratory flow rates, demonstrating a 28% improvement in dose delivery efficiency while reducing medication wastage in clinical settings.”
The Aerosol Bronchodilator Market is evolving rapidly due to increasing respiratory disease prevalence, ongoing technological innovation, and the adoption of smart inhalation devices across global healthcare systems. The rising urban pollution levels and the growth of the elderly population with chronic respiratory conditions are expanding demand within the Aerosol Bronchodilator Market. Regulatory enhancements promoting eco-friendly propellants in aerosol formulations are influencing production strategies, while the demand for advanced drug delivery devices that enhance medication adherence is growing. Market players are focusing on the development of portable and digitally integrated inhalers, and the expansion of online pharmacy distribution channels is further influencing the competitive landscape within the Aerosol Bronchodilator Market, ensuring accessibility in emerging economies.
Rising cases of asthma, COPD, and bronchospasm globally are a significant driver for the Aerosol Bronchodilator Market, fueling consistent demand for effective and rapid-onset inhalation therapies. The World Health Organization estimates that over 262 million people globally suffer from asthma, leading to high usage of aerosol bronchodilators for daily management and emergency care. Increased urbanization and industrial pollution contribute to higher incidences of respiratory conditions, necessitating advanced bronchodilator solutions. The adoption of aerosol-based drug delivery systems within emergency care units and home healthcare setups has further increased, driven by their ease of use and quick therapeutic action, boosting the demand within the Aerosol Bronchodilator Market.
Stringent environmental regulations regarding the use of hydrofluoroalkane-based propellants in aerosol inhalers are posing operational challenges for manufacturers within the Aerosol Bronchodilator Market. Regulatory bodies in Europe and North America are imposing stricter guidelines to reduce the carbon footprint of medical aerosols, requiring market players to invest in research for eco-friendly propellant alternatives. The transition to low-GWP (Global Warming Potential) propellants increases manufacturing costs and demands reformulation, extending product development timelines. These environmental compliance requirements are creating a financial and operational burden for small and medium-sized manufacturers, limiting their capacity to enter or expand within the Aerosol Bronchodilator Market.
The integration of digital technologies, including Bluetooth connectivity and dose tracking sensors in inhalers, presents a significant growth opportunity for the Aerosol Bronchodilator Market. Smart inhalers with adherence monitoring and data analytics capabilities are gaining traction in hospitals and home healthcare, enabling clinicians to track patient medication usage accurately. Digital inhalers have shown a reduction in emergency visits by ensuring timely bronchodilator usage, enhancing treatment outcomes in asthma and COPD management. The demand for personalized respiratory care and telehealth-compatible inhalation devices is rising, creating new avenues for market players to innovate and expand within the Aerosol Bronchodilator Market.
The high cost associated with advanced aerosol inhalers embedded with smart technology remains a challenge within the Aerosol Bronchodilator Market. These devices, equipped with sensors and connectivity features for tracking dosage and usage patterns, can be expensive for patients in low- and middle-income regions, limiting widespread adoption. Additionally, training and educating healthcare providers and patients on the usage of these technologically advanced devices require structured programs, adding operational costs for healthcare systems. The initial investment for adopting advanced inhalers and transitioning from conventional models to smart devices also poses budgetary challenges for hospitals, restraining market penetration in cost-sensitive markets within the Aerosol Bronchodilator Market.
• Adoption of Smart Inhalers and Connected Devices: The Aerosol Bronchodilator market is witnessing a notable shift toward smart inhalers equipped with sensors that track medication usage, inhalation patterns, and patient adherence. These devices enable real-time data sharing with healthcare providers, enhancing personalized care in asthma and COPD management. In 2025, over 3 million units of Bluetooth-enabled aerosol inhalers are projected to be in active patient use globally, indicating the growing integration of digital health in respiratory care and transforming monitoring and treatment effectiveness in clinical and homecare settings.
• Shift Toward Environmentally Friendly Propellants: The market is experiencing a clear trend toward eco-friendly propellant systems in aerosol bronchodilators, aligning with stricter environmental regulations. Manufacturers are actively transitioning from traditional hydrofluoroalkane-based propellants to lower global warming potential (GWP) alternatives, reducing environmental footprints. Several leading manufacturers have announced pilot production lines for low-GWP propellant-based bronchodilators, targeting full-scale rollout in high-demand regions by 2026 to meet compliance goals while maintaining drug delivery efficiency.
• Increased Demand for Portable and Compact Devices: There is a rising demand for compact, lightweight aerosol bronchodilators to support mobility and patient convenience, particularly for the aging population and individuals with chronic respiratory diseases. Portable inhalers with easy-to-use mechanisms are gaining preference in home healthcare and outpatient care setups. Surveys indicate that over 60% of patients prefer portable aerosol bronchodilators, driving manufacturers to focus on ergonomic and patient-friendly designs without compromising on medication effectiveness.
• Integration of AI in Manufacturing and Quality Control: AI and machine learning integration within production processes are improving quality control in aerosol bronchodilator manufacturing. Automated systems are reducing error rates in dose calibration while enhancing the consistency of aerosol distribution. Facilities adopting AI-driven predictive maintenance have reported a 20% reduction in downtime, contributing to increased production efficiency and the ability to meet the growing global demand for aerosol bronchodilators effectively.
The Aerosol Bronchodilator market segmentation provides a structured understanding of market behavior, covering type, application, and end-user perspectives to guide business decisions. Types of aerosol bronchodilators include short-acting and long-acting variants, with technological advancements driving niche innovations within each. Applications in asthma, COPD, and other respiratory disorders define the utilization landscape, with asthma management dominating in volume usage while COPD care reflects a fast adoption of digital inhalers. End-users such as hospitals, home healthcare settings, and specialty clinics display distinct consumption patterns, with hospitals leading the adoption of advanced aerosol delivery devices and home healthcare rapidly growing due to patient-centric care preferences and expanded home monitoring capabilities. This segmentation reveals areas of stable demand and identifies growth opportunities aligned with evolving healthcare delivery models.
The Aerosol Bronchodilator market encompasses various types, including short-acting beta-agonists (SABAs), long-acting beta-agonists (LABAs), anticholinergics, combination bronchodilators, and corticosteroid-containing inhalers. Short-acting beta-agonists lead the segment due to their immediate relief action, making them the primary choice for acute asthma attacks and sudden bronchospasm, supported by high prescription rates in emergency care. Long-acting beta-agonists are the fastest-growing type, driven by rising COPD prevalence and the need for sustained bronchodilation in chronic management, with increasing physician preference for maintenance therapy devices. Anticholinergics and combination bronchodilators are steadily gaining traction, offering synergistic effects for advanced respiratory care. Corticosteroid-containing inhalers hold niche relevance for patients requiring anti-inflammatory and bronchodilation effects combined, addressing complex respiratory conditions. The diversity in types ensures tailored treatment pathways, improving patient outcomes across multiple respiratory conditions while supporting the market’s adaptability to varied healthcare settings.
The Aerosol Bronchodilator market primarily serves applications in asthma management, COPD care, and acute bronchospasm treatment. Asthma management remains the leading application due to the global rise in asthma cases, with millions of patients relying on aerosol bronchodilators for daily symptom control and emergency relief. COPD care is the fastest-growing application, driven by aging populations and lifestyle-related respiratory issues, with increased awareness and screening initiatives boosting treatment uptake globally. Acute bronchospasm treatment in emergency settings highlights the utility of fast-acting aerosol bronchodilators, ensuring rapid response during critical respiratory distress situations. Other niche applications include their use in exercise-induced bronchospasm and postoperative respiratory care, expanding the utility of aerosol bronchodilators beyond chronic disease management. The diversity in application areas allows manufacturers to cater to multiple segments within healthcare, from hospitals to home settings, reinforcing the integral role of aerosol bronchodilators in comprehensive respiratory care.
The Aerosol Bronchodilator market’s end-user landscape includes hospitals, home healthcare, and specialty clinics, each contributing distinctly to market demand. Hospitals are the leading end-user segment, driven by consistent use in emergency departments and respiratory care units for acute and chronic respiratory disease management, with hospitals adopting advanced inhalation devices for accurate dosing and rapid interventions. Home healthcare is the fastest-growing end-user segment due to increasing patient preference for self-management of asthma and COPD, supported by the availability of easy-to-use, portable aerosol bronchodilators and remote monitoring solutions. Specialty clinics play a crucial role in the market by providing targeted respiratory therapies and personalized patient management plans, driving the adoption of advanced inhalation therapies in outpatient settings. This end-user segmentation illustrates the market’s alignment with patient-centric healthcare models and the demand for accessibility and convenience across different care environments.
North America accounted for the largest market share at 42% in 2024 however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 7.8% between 2025 and 2032.
North America’s dominance in the Aerosol Bronchodilator Market is driven by its advanced healthcare infrastructure, widespread use of smart inhalers, and consistent demand across hospital and home healthcare settings. Europe, holding around 28% market share, demonstrates steady adoption due to strong regulatory frameworks and an increasing patient base requiring chronic respiratory care. The Asia-Pacific region, led by China, India, and Japan, is experiencing rapid urbanization and rising respiratory disease incidence, increasing the uptake of aerosol bronchodilators across expanding healthcare systems. South America and the Middle East & Africa are displaying emerging demand, supported by gradual healthcare modernization and urban pollution challenges, positioning these regions as potential growth areas within the Aerosol Bronchodilator Market during the forecast period.
Smart Inhaler Integration Accelerating Market Penetration
North America holds a commanding 42% share in the Aerosol Bronchodilator Market, driven by the widespread use of inhalation therapies for asthma and COPD management across the United States and Canada. Key industries fueling this demand include hospital respiratory care units and home healthcare, where advanced bronchodilator devices are integral for emergency and chronic treatment. Regulatory support for eco-friendly propellant transitions and FDA fast-track approvals for inhalation therapies are streamlining product launches. The region is witnessing significant technological advancements with AI-enabled inhalers, Bluetooth connectivity for adherence monitoring, and portable inhaler designs improving patient convenience and compliance within the Aerosol Bronchodilator Market.
Technological Innovations Strengthening Respiratory Care Delivery
Europe accounts for approximately 28% of the Aerosol Bronchodilator Market, with Germany, the UK, and France leading in adoption due to a high burden of chronic respiratory diseases and well-established healthcare systems. Regulatory bodies such as the EMA are encouraging sustainable inhalation technologies, pushing manufacturers toward low-GWP propellants and recyclable inhaler components. The region has seen accelerated adoption of digital dose-tracking inhalers and smart monitoring devices within clinical and homecare environments. Emerging technologies, including AI-powered respiratory monitoring and personalized inhaler solutions, are enhancing treatment precision, reinforcing Europe’s position in advancing effective respiratory care within the Aerosol Bronchodilator Market.
Growing Healthcare Access Driving Device Uptake
Asia-Pacific ranks as the fastest-growing region in the Aerosol Bronchodilator Market, with China, India, and Japan representing the top consuming countries due to their large patient populations and rising incidence of asthma and COPD. Healthcare infrastructure development and expanding insurance coverage in these countries are improving access to inhalation therapies. The region is also witnessing the establishment of local manufacturing units for aerosol bronchodilators, supporting affordability and timely supply. Innovation hubs in Japan and China are promoting the development of portable, digitally connected inhalers, while local initiatives for urban pollution control are indirectly boosting the demand for bronchodilator devices in the Aerosol Bronchodilator Market.
Healthcare Modernization Supporting Market Expansion
Brazil and Argentina are leading the South American Aerosol Bronchodilator Market, contributing to a combined regional market share of approximately 8% in 2024. Brazil’s growing investment in public healthcare infrastructure and respiratory disease management programs is increasing demand for aerosol bronchodilators in hospitals and outpatient care. The region’s ongoing expansion of urban healthcare facilities and improvements in medical supply chains are ensuring better availability of advanced inhalation therapies. Government incentives for local pharmaceutical manufacturing and regional trade agreements are supporting the growth of the Aerosol Bronchodilator Market across South America while enhancing accessibility to essential respiratory care products.
Healthcare Expansion Driving Respiratory Device Demand
The Middle East & Africa region is displaying emerging demand within the Aerosol Bronchodilator Market, with the UAE and South Africa leading growth due to increasing investments in healthcare modernization and respiratory disease management initiatives. The region is witnessing a rising prevalence of asthma and COPD, driving the need for accessible bronchodilator solutions across hospitals and home healthcare settings. Technological modernization, including the adoption of portable inhalation devices, is gaining traction in private healthcare facilities. Local regulatory agencies are promoting public-private partnerships and facilitating smoother market entry for inhalation therapy products, strengthening the region’s role in the expanding Aerosol Bronchodilator Market.
United States – 38% market share: Driven by advanced healthcare infrastructure, high patient demand for inhalation therapies, and widespread smart inhaler adoption within the Aerosol Bronchodilator Market.
China – 15% market share: Supported by large-scale manufacturing capacity, rising urban pollution-linked respiratory diseases, and rapid healthcare system expansion fueling demand in the Aerosol Bronchodilator Market.
The Aerosol Bronchodilator market is characterized by a competitive environment with over 50 active global and regional players focusing on product innovation, technological integration, and geographic expansion. Leading manufacturers are strategically enhancing their market positioning through the development of smart inhalers equipped with dose tracking and Bluetooth connectivity, addressing the demand for advanced respiratory care solutions. Partnerships between pharmaceutical companies and technology firms are increasing to co-develop AI-enabled inhalation devices that improve patient adherence and monitoring. Recent product launches include eco-friendly inhalers utilizing low-GWP propellants, reflecting the market’s responsiveness to sustainability initiatives. Mergers and acquisitions are being pursued to strengthen R&D capabilities and expand production capacities, particularly in fast-growing regions like Asia-Pacific. Companies are also leveraging digital supply chain models and direct-to-patient delivery networks to enhance market reach. This active competitive landscape is fostering continuous innovation within the Aerosol Bronchodilator market while supporting diverse patient needs in hospital, outpatient, and home healthcare environments.
GlaxoSmithKline plc
AstraZeneca plc
Boehringer Ingelheim International GmbH
Teva Pharmaceutical Industries Ltd.
Cipla Limited
Novartis AG
Chiesi Farmaceutici S.p.A.
Orion Corporation
Sunovion Pharmaceuticals Inc.
Mylan N.V.
Technological advancements are reshaping the Aerosol Bronchodilator market by improving inhaler efficiency, patient adherence, and environmental sustainability. Smart inhalers equipped with sensors and Bluetooth connectivity enable real-time monitoring of dosage and inhalation technique, reducing medication wastage and enhancing patient outcomes. It is estimated that by 2025, over 3 million smart inhalers will be in active patient use globally, reflecting rapid digital integration in respiratory care.
The shift toward low-GWP propellants in aerosol inhalers is addressing environmental concerns while maintaining effective drug delivery. Manufacturers have implemented advanced valve technologies and precision dose counters to enhance consistency in aerosol delivery. Automation in manufacturing lines using AI-based quality control systems is reducing product defects and increasing operational efficiency, with some facilities reporting a 20% reduction in downtime through predictive maintenance.
Additionally, integration of data analytics platforms is enabling healthcare providers to track patient usage patterns, facilitating personalized treatment adjustments. Hybrid inhaler models combining corticosteroids with bronchodilators in a single device are gaining traction for patients requiring comprehensive respiratory management. The industry is also exploring the use of biodegradable inhaler components, aligning with global sustainability goals. Collectively, these technologies are elevating the effectiveness, usability, and environmental compatibility of aerosol bronchodilators in healthcare systems worldwide.
• In February 2024, AstraZeneca launched an AI-enabled smart inhaler designed for COPD patients, featuring real-time adherence monitoring and inhalation tracking, demonstrating a 25% improvement in medication adherence during initial pilot programs across the UK.
• In September 2023, Cipla introduced a portable, eco-friendly aerosol bronchodilator using low-GWP propellants across its India and Southeast Asia operations, targeting a 30% reduction in environmental impact while maintaining high delivery efficiency.
• In April 2024, GlaxoSmithKline unveiled a connected digital inhaler with Bluetooth-based dose tracking and data sharing for hospital respiratory care units in the US, enhancing patient monitoring and reducing emergency department visits among high-risk asthma patients.
• In November 2023, Boehringer Ingelheim expanded its aerosol bronchodilator manufacturing facility in Germany, adding advanced automated production lines to increase annual output capacity by 20%, addressing rising demand in European and Asian markets.
The Aerosol Bronchodilator Market Report comprehensively analyzes the global landscape covering product types, applications, regional markets, and technological trends influencing growth and strategic decisions. It includes in-depth insights on short-acting and long-acting bronchodilators, anticholinergics, combination inhalers, and corticosteroid-containing aerosol devices, providing a clear understanding of product utilization patterns and clinical preferences across multiple healthcare environments.
The report examines key applications such as asthma, COPD, and acute bronchospasm treatment within hospital, home healthcare, and outpatient settings, supported by regional consumption data across North America, Europe, Asia-Pacific, South America, and the Middle East & Africa. It further explores the impact of digital health integration, including smart inhalers with sensor-based monitoring, and the transition toward eco-friendly propellant systems aligning with sustainability initiatives.
Detailed competitive analysis highlights major industry players, their innovation strategies, and partnerships driving product development. The scope also covers emerging trends in manufacturing automation, AI-driven quality control, and personalized respiratory care initiatives, capturing niche segments such as portable and connected inhaler devices. This structured and data-focused analysis enables business leaders and industry professionals to navigate opportunities, assess risk factors, and develop effective strategies within the evolving Aerosol Bronchodilator market landscape.
| Report Attribute/Metric | Report Details |
|---|---|
|
Market Revenue in 2024 |
USD 8,517 Million |
|
Market Revenue in 2032 |
USD 14,308.76 Million |
|
CAGR (2025 - 2032) |
6.7% |
|
Base Year |
2024 |
|
Forecast Period |
2025 - 2032 |
|
Historic Period |
2020 - 2024 |
|
Segments Covered |
By Types
By Application
By End-User
|
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Region Covered |
North America, Europe, Asia-Pacific, South America, Middle East, Africa |
|
Key Players Analyzed |
GlaxoSmithKline plc, AstraZeneca plc, Boehringer Ingelheim International GmbH, Teva Pharmaceutical Industries Ltd., Cipla Limited, Novartis AG, Chiesi Farmaceutici S.p.A., Orion Corporation, Sunovion Pharmaceuticals Inc., Mylan N.V. |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
