Reports
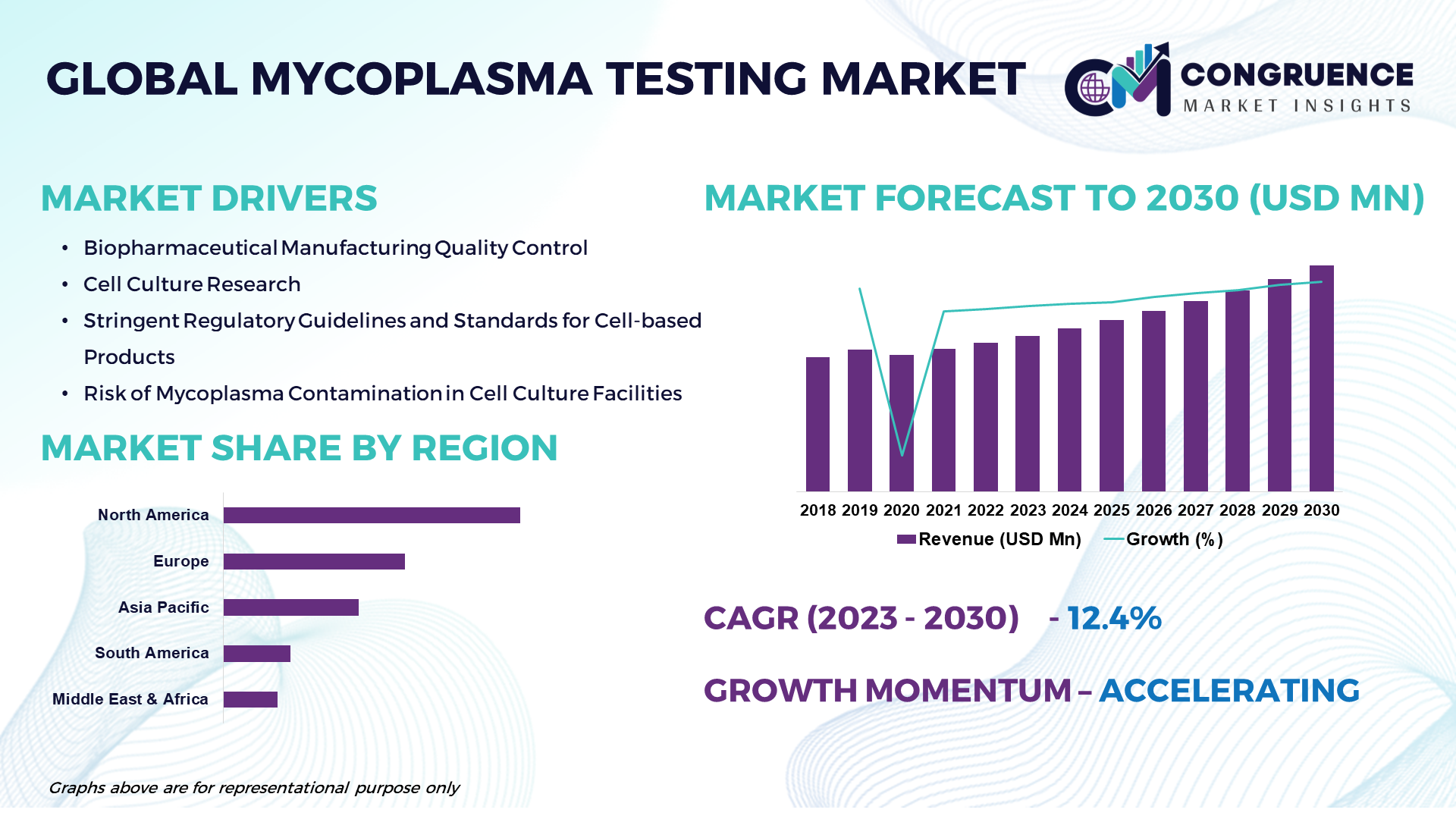
The Global Mycoplasma Testing Market is projected to expand at a robust CAGR of 12.4% from 2023 to 2030, driven by factors such as technological advancements in mycoplasma testing, the rising prevalence of mycoplasma contamination, and the growing emphasis on quality control in biopharmaceutical manufacturing. Mycoplasma testing is a critical aspect of quality control in biopharmaceutical manufacturing and cell-based research. Mycoplasma contamination poses a significant risk to cell culture integrity, leading to inaccurate experimental results, compromised product quality, and potential safety concerns for biopharmaceutical products. Mycoplasma testing involves the detection and quantification of mycoplasma species in cell culture samples using various molecular and microbiological methods. The Mycoplasma Testing Market is witnessing substantial growth due to the increasing awareness about mycoplasma contamination, stringent regulatory guidelines for cell-based products, and the rising demand for reliable mycoplasma detection methods in biopharmaceutical production. Key factors driving market growth include the expansion of the biopharmaceutical industry, the growing adoption of cell-based therapies, and the increasing investment in research and development activities.
Mycoplasma Testing Market Major Driving Forces
Biopharmaceutical Manufacturing Quality Control: The increasing emphasis on quality control and assurance in biopharmaceutical manufacturing is driving the demand for mycoplasma testing solutions to ensure the safety, purity, and efficacy of cell-based products and biopharmaceuticals.
Cell Culture Research: The growing adoption of cell culture techniques in academic research, drug discovery, and regenerative medicine has led to an increased need for mycoplasma testing to maintain the integrity and reproducibility of cell-based experiments and research findings.
Regulatory Compliance: Stringent regulatory guidelines and standards for cell-based products, biopharmaceuticals, and cell therapy products mandate the implementation of mycoplasma testing as a crucial step in bioprocessing and product release testing to meet regulatory requirements and ensure product safety and quality.
Risk Mitigation: The risk of mycoplasma contamination in cell culture facilities, biopharmaceutical production plants, and research laboratories poses significant challenges to product development, manufacturing processes, and experimental outcomes, driving the demand for mycoplasma testing solutions to mitigate contamination risks and ensure data integrity.
Market Expansion: The expansion of the biopharmaceutical industry, the growing adoption of cell-based therapies, and the increasing investment in research and development activities are driving market growth by creating opportunities for mycoplasma testing solution providers to cater to the growing demand for reliable and efficient mycoplasma detection methods.
Mycoplasma Testing Market Key Opportunities
Automated Testing Solutions: The growing demand for high-throughput, automated mycoplasma testing solutions presents opportunities for manufacturers to develop innovative instruments and assay platforms that offer rapid, accurate, and cost-effective mycoplasma detection and quantification capabilities for biopharmaceutical manufacturing and cell culture research applications.
Point-of-Care Testing: The emergence of point-of-care mycoplasma testing devices and portable diagnostic platforms presents opportunities for market players to develop compact, user-friendly testing kits and instruments that enable on-site mycoplasma testing in cell culture facilities, biopharmaceutical production plants, and research laboratories for real-time contamination monitoring and rapid decision-making.
Technological Advancements: The continuous advancement of molecular and microbiological techniques, such as polymerase chain reaction (PCR), enzyme-linked immunosorbent assay (ELISA), direct assay, and indirect assay, presents opportunities for technology developers to enhance the sensitivity, specificity, and reliability of mycoplasma testing methods for improved detection and quantification of mycoplasma species in cell culture samples.
Regulatory Support: The support and endorsement of regulatory agencies and international organizations for standardized mycoplasma testing methods, quality control guidelines, and regulatory frameworks for biopharmaceutical manufacturing and cell-based research present opportunities for market players to align their products and services with regulatory requirements and gain market approval and acceptance.
Collaborative Partnerships: Collaborative partnerships and strategic alliances between mycoplasma testing solution providers, biopharmaceutical companies, contract research organizations, and academic research institutes present opportunities for joint research and development initiatives, technology transfer agreements, and collaborative studies to address unmet needs and challenges in mycoplasma testing and quality control in biopharmaceutical manufacturing and cell culture research.
Mycoplasma Testing Market Key Trends
· PCR-Based Assays: The increasing adoption of polymerase chain reaction (PCR)-based assays for mycoplasma testing, including endpoint PCR, real-time PCR, and droplet digital PCR (ddPCR), due to their high sensitivity, specificity, and rapid turnaround time for mycoplasma detection and quantification in cell culture samples.
· ELISA-Based Assays: The growing use of enzyme-linked immunosorbent assay (ELISA)-based assays for mycoplasma testing, including direct ELISA and indirect ELISA, for antibody detection and antigen quantification in cell culture supernatants and lysates, providing a reliable alternative to PCR-based methods for mycoplasma detection in biopharmaceutical manufacturing and research laboratories.
· Automation and Robotics: The increasing integration of automation and robotics into mycoplasma testing workflows, including liquid handling systems, robotic platforms, and high-throughput screening instruments, to streamline sample processing, improve assay accuracy, and enhance workflow efficiency in biopharmaceutical production and cell culture research applications.
· Multiplexing Technologies: The development of multiplexing technologies for simultaneous detection and quantification of multiple mycoplasma species in cell culture samples using a single assay platform, enabling comprehensive screening and identification of contaminating microorganisms in biopharmaceutical manufacturing and research laboratories.
· Next-Generation Sequencing: The emergence of next-generation sequencing (NGS) technologies for mycoplasma testing, including metagenomic sequencing, shotgun sequencing, and amplicon sequencing, for comprehensive analysis of microbial communities and genetic diversity in cell culture samples, providing insights into mycoplasma contamination and microbial ecology in biopharmaceutical manufacturing and cell culture research.
Market Competition Landscape
The global mycoplasma testing market is characterized by intense competition among a diverse range of market players offering a wide array of mycoplasma testing products and services to cater to the growing demand for reliable and efficient mycoplasma detection solutions in biopharmaceutical manufacturing and cell culture research applications. Prominent players in the Mycoplasma Testing Market include:
· Thermo Fisher Scientific Inc.
· Merck KGaA
· Lonza Group Ltd.
· Charles River Laboratories International, Inc.
· PromoCell GmbH
· Biological Industries Israel Beit Haemek Ltd.
· American Type Culture Collection (ATCC)
· Sartorius AG
· Bionique Testing Laboratories, Inc.
· Minerva Biolabs GmbH
These companies offer a comprehensive portfolio of mycoplasma testing kits, reagents, instruments, and services for cell line testing, virus testing, and end-of-production cell testing in biopharmaceutical manufacturing and cell culture research applications.
|
Report Attribute/Metric |
Details |
|
Base Year |
2022 |
|
Forecast Period |
2023 – 2030 |
|
Historical Data |
2018 to 2022 |
|
Forecast Unit |
Value (US$ Mn) |
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Segments Covered |
· By Product Type (Kits & Reagents, Instruments) · By Technology (PCR, ELISA, Direct Assay, Indirect Assay) · By Application (Cell Line Testing, Virus Testing, End of Production Cells Testing) · By End-user (Pharmaceutical & Biotechnology Companies, Contract Research Organizations, Academic Research Institutes) |
|
Geographies Covered |
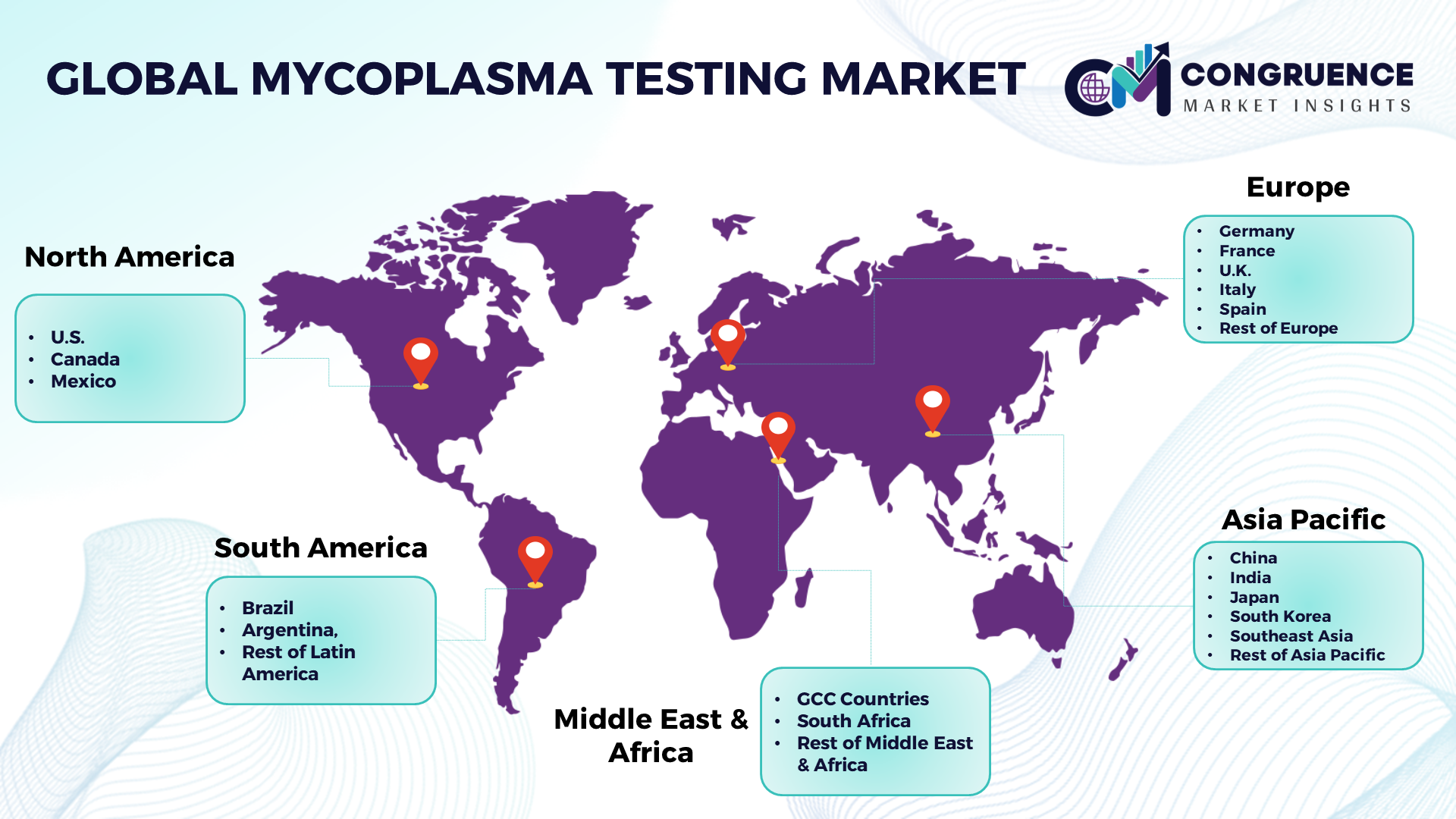
North America: U.S., Canada and Mexico Europe: Germany, France, U.K., Italy, Spain, and Rest of Europe Asia Pacific: China, India, Japan, South Korea, Southeast Asia, and Rest of Asia Pacific South America: Brazil, Argentina, and Rest of Latin America Middle East & Africa: GCC Countries, South Africa, and Rest of Middle East & Africa |
|
Key Players Analyzed |
Thermo Fisher Scientific Inc., Merck KGaA, Lonza Group Ltd., Charles River Laboratories International, Inc., PromoCell GmbH, Biological Industries Israel Beit Haemek Ltd., American Type Culture Collection (ATCC), Sartorius AG, Bionique Testing Laboratories, Inc., and Minerva Biolabs GmbH |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
