Reports
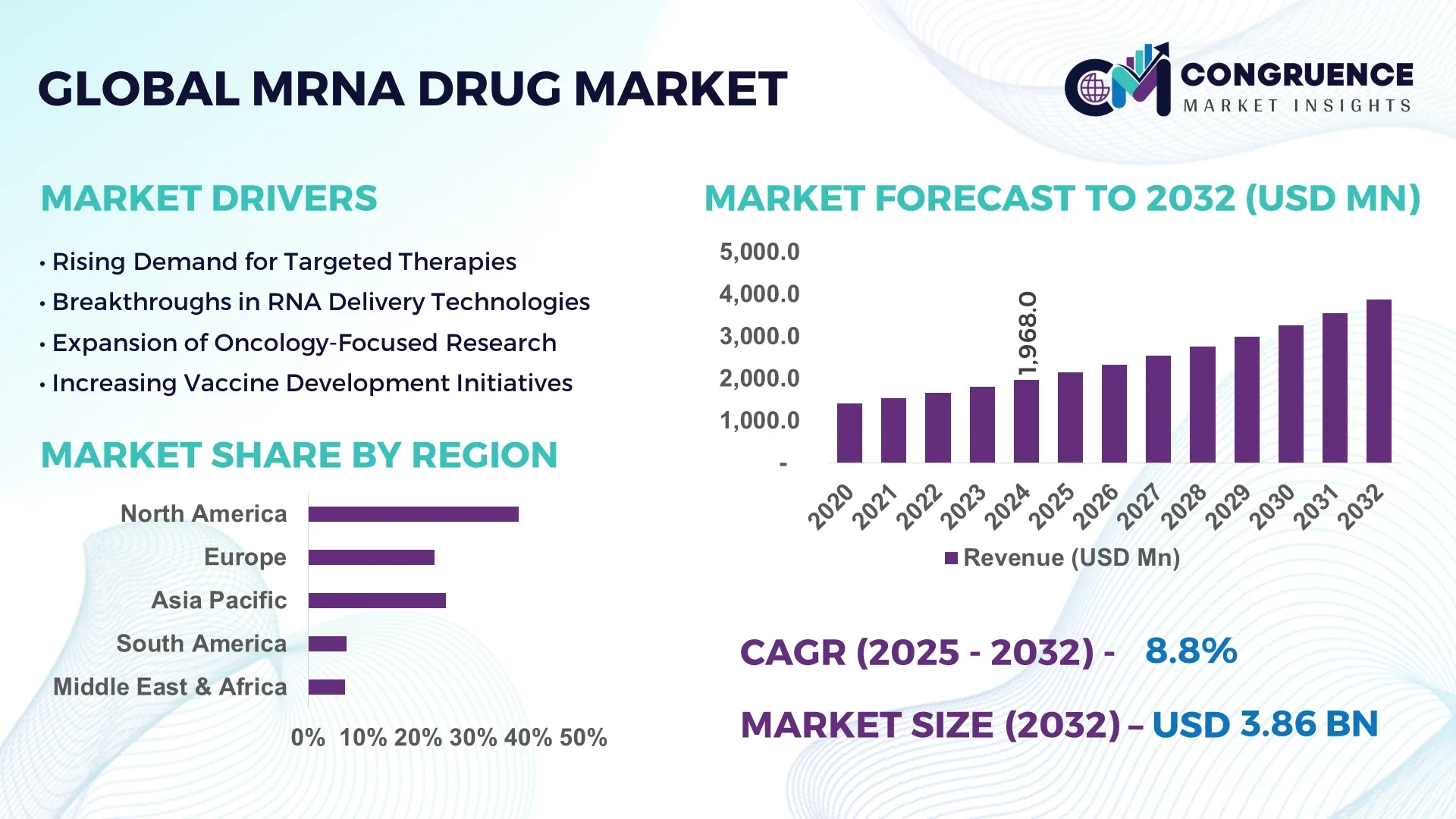
The Global mRNA Drug Market was valued at USD 1,968.0 Million in 2024 and is anticipated to reach a value of USD 3,869.9 Million by 2032 expanding at a CAGR of 8.8% between 2025 and 2032.
To Learn More About This Report, Request A Free Sample Copy
The United States dominates the marketplace, driven by significant investments in biotechnology research, a robust healthcare infrastructure, and a strong presence of leading pharmaceutical companies developing innovative mRNA drug therapies. Government support through funding and regulatory incentives further accelerates market growth in this region.
Globally, the mRNA drug market is witnessing rapid expansion fueled by advances in mRNA technology and its applications in treating infectious diseases, oncology, and rare genetic disorders. Increasing awareness about personalized medicine and the development of novel delivery systems enhance the therapeutic efficacy of mRNA drugs. Additionally, collaborations between biotech firms and research institutes are fostering innovation, while expanding clinical trials continue to validate the safety and effectiveness of mRNA-based treatments, bolstering market confidence.
Artificial Intelligence (AI) is profoundly transforming the mRNA drug market by accelerating drug discovery, optimizing molecular design, and enhancing personalized treatment strategies. AI-driven algorithms analyze vast datasets from genomic sequences, patient profiles, and clinical trial results, enabling researchers to identify potential mRNA targets more quickly and accurately than traditional methods. This improves the efficiency of drug candidate selection and reduces the time to market.
Moreover, AI assists in optimizing mRNA delivery systems, such as lipid nanoparticles, by predicting the most effective formulations and reducing adverse effects. Machine learning models simulate biological interactions and immune responses, allowing developers to tailor mRNA constructs to individual patients, thereby advancing personalized medicine.
AI-powered platforms also streamline clinical trial design and patient recruitment by identifying suitable candidates based on genetic markers and health data, increasing the success rates of trials. Additionally, real-time monitoring and predictive analytics help in assessing treatment outcomes and managing side effects more effectively.
The integration of AI in manufacturing processes further ensures consistency, quality control, and scalability of mRNA drugs. With continuous advancements in AI technology, the mRNA drug market is set to benefit from more innovative, safer, and targeted therapies, addressing unmet medical needs across various therapeutic areas.
“In 2024, a leading biotech company implemented an AI-based platform that reduced the mRNA drug candidate identification process by 60%, enabling faster progression from research to clinical trials and significantly cutting development costs.”
The growing demand for targeted and personalized therapeutics is driving the mRNA drug market. Patients and healthcare providers seek treatments that offer higher efficacy and fewer side effects compared to traditional drugs. mRNA drugs provide the ability to encode specific proteins, enabling precise intervention at the molecular level, which is highly advantageous for complex diseases such as cancer and genetic disorders.
The production of mRNA drugs requires sophisticated technology and stringent conditions, contributing to high manufacturing costs. Additionally, mRNA therapeutics often require ultra-cold storage, which limits distribution, especially in low-resource settings. These factors pose challenges to widespread adoption and affordability of mRNA drugs globally.
Beyond COVID-19, the mRNA platform presents substantial opportunities in developing vaccines and therapeutics for other infectious diseases like influenza, cytomegalovirus, and Zika virus. Ongoing research and increasing government support are fostering the development of new mRNA vaccines that can be rapidly adapted for emerging pathogens.
Navigating the complex regulatory landscape for mRNA drugs remains a challenge. Regulatory agencies require extensive safety and efficacy data, which can prolong approval timelines. Additionally, gaining reimbursement and market access, especially in emerging economies, is difficult due to limited infrastructure and budget constraints, restricting patient reach.
Strategic Collaborations and Partnerships: The market is witnessing increased collaborations between pharmaceutical companies and technology providers. These partnerships focus on combining mRNA technology with innovative delivery systems to improve drug development efficiency and therapeutic outcomes.
Advancements in Lipid Nanoparticle (LNP) Delivery Systems: Improved LNP formulations are enhancing mRNA delivery efficiency by increasing stability, reducing required dosages, and minimizing side effects. This advancement plays a key role in boosting patient compliance and expanding therapeutic uses beyond vaccines.
Growth of mRNA Therapeutics in Oncology: Several mRNA-based cancer therapies are advancing into late-stage clinical trials. These treatments leverage mRNA’s ability to stimulate immune responses specifically targeted at tumor cells, offering promising alternatives to traditional cancer therapies.
Emergence of Asia-Pacific as a Key Market: Increasing government investments, expanding clinical trial activities, and improving healthcare infrastructure in the Asia-Pacific region are driving its rise as a significant contributor to the global mRNA drug market growth.
The mRNA drug market is broadly segmented by type, application, and end-user, each offering unique insights into the growth dynamics of the industry. Understanding these segments provides a clearer picture of demand patterns, technological advances, and investment focus areas. Different types of mRNA drugs cater to diverse therapeutic needs, while applications range from infectious diseases to oncology and rare genetic disorders. End-users primarily include hospitals, research institutes, and pharmaceutical companies, each playing a crucial role in the commercialization and adoption of mRNA therapies. This segmentation enables stakeholders to identify lucrative opportunities and tailor strategies to target specific market demands effectively.
The mRNA drug market is primarily divided into modified mRNA and non-modified mRNA types. Modified mRNA dominates the market due to its enhanced stability, reduced immunogenicity, and improved translation efficiency, making it the preferred choice for vaccines and therapeutics. This segment accounts for a significant share of ongoing clinical trials and commercial products. Non-modified mRNA, while less stable, is gaining traction due to simpler manufacturing processes and cost advantages, representing the fastest-growing segment as improvements in formulation continue. Additionally, self-amplifying mRNA, a sub-segment, is attracting attention for its ability to produce higher protein yields with lower doses, potentially lowering treatment costs and improving patient compliance. The growing interest in personalized medicine further fuels demand for these diverse mRNA types across therapeutic areas.
In terms of applications, infectious diseases lead the mRNA drug market, driven by the global focus on vaccine development following the COVID-19 pandemic. This segment remains the largest due to widespread immunization efforts and ongoing research into vaccines for influenza, Zika, and cytomegalovirus. Oncology is the fastest-growing application segment, propelled by advances in cancer immunotherapy that harness mRNA technology to stimulate targeted immune responses against tumors. Rare genetic disorders also represent a critical application area, where mRNA therapies offer hope for conditions previously deemed untreatable. The ability of mRNA drugs to encode specific proteins allows for tailored treatments across these diverse disease categories, broadening market scope and investment.
Hospitals and healthcare facilities constitute the largest end-user segment of the mRNA drug market, as they are the primary points of administration for vaccines and therapeutic treatments. This segment benefits from expanding healthcare infrastructure and increased awareness about mRNA therapies among healthcare professionals and patients. Research institutes and academic organizations are the fastest-growing end-user segment, driven by rising investments in R&D and clinical trials aimed at discovering novel mRNA applications. Pharmaceutical and biotechnology companies also play a pivotal role by focusing on product development, manufacturing, and commercialization activities. Collaborations between these end-users facilitate innovation and faster market entry of mRNA drugs, underpinning overall market growth.
North America accounted for the largest market share at 38.3% in 2024; however, the Asia-Pacific region is expected to register the fastest growth, expanding at a CAGR of 9.5% between 2025 and 2032.
To Learn More About This Report, Request A Free Sample Copy
North America leads due to its advanced healthcare infrastructure, high R&D expenditure, and strong presence of biotechnology firms specializing in mRNA therapeutics. Meanwhile, Asia-Pacific's rapid growth is driven by increasing government investments, expanding clinical trial activities, and growing awareness of mRNA-based treatments in emerging economies such as China and India. Europe also holds a significant share with increasing focus on personalized medicine and supportive regulatory frameworks enhancing market adoption.
Innovation and Strong Industry Presence Propel Growth
North America continues to lead the mRNA drug market, supported by substantial biotech investments and a well-established pharmaceutical ecosystem. The U.S. contributes significantly with numerous ongoing clinical trials and several mRNA vaccines and therapies already commercially available. Canada is increasingly focusing on expanding research capacities in this space. The region benefits from cutting-edge AI integration for drug development and delivery system advancements. Furthermore, strong government funding and collaborations between academia and industry accelerate the development of next-generation mRNA therapeutics. Regulatory support and rapid adoption in healthcare systems bolster market expansion here.
Rising Adoption of Personalized Medicine and Regulatory Support
Europe maintains a strong position with increasing adoption of mRNA drugs across oncology and rare disease treatments. Countries like Germany, France, and the UK have invested heavily in mRNA research infrastructure and clinical trials. The region’s stringent but supportive regulatory environment encourages innovation and ensures high-quality standards for new mRNA drugs. Public-private partnerships are fostering the translation of research into viable products. Additionally, Europe's growing emphasis on precision medicine is driving demand for mRNA therapies tailored to genetic profiles, particularly in cancer treatment and autoimmune diseases.
Rapid Expansion Driven by Investment and Infrastructure Development
Asia-Pacific is witnessing remarkable growth in the mRNA drug market, primarily led by China and India. Increasing government initiatives and funding support biotechnology startups and research centers focused on mRNA technology. The region benefits from a large patient population, expanding healthcare infrastructure, and rising awareness of novel therapeutics. Clinical trials for mRNA vaccines and therapies are growing rapidly, especially in infectious diseases and oncology segments. Additionally, manufacturing capabilities are improving, positioning Asia-Pacific as a major player in global mRNA drug production and distribution networks.
Emerging Market with Growing Healthcare Investment
South America, led by Brazil and Argentina, is emerging as a key market for mRNA drugs, supported by increasing healthcare expenditure and growing interest in biotechnology. While the market size is smaller compared to North America and Europe, there is rising adoption of mRNA vaccines for infectious diseases, especially following global vaccination drives. Efforts to enhance clinical research infrastructure and collaboration with global pharma companies are accelerating access to mRNA therapies. The region is expected to witness steady growth as awareness and affordability improve.
Building Foundations for Future Growth
The Middle East & Africa region is in the early stages of mRNA drug market development, with countries like the UAE and South Africa taking the lead. Investments in biotechnology research, healthcare infrastructure improvements, and government initiatives to diversify healthcare offerings are notable trends. The region shows increasing participation in global clinical trials and growing interest in mRNA vaccines to address infectious disease challenges. While market penetration remains limited, expanding collaborations with international biotech firms and gradual regulatory framework development are key drivers for future market potential.
United States - It holds the highest market share at approximately USD 750 million in 2024. It benefits from advanced biotechnology research and development facilities and is home to numerous commercially available mRNA vaccines and therapeutics. Strong government funding and collaborations between academia and industry accelerate innovation, making the U.S. a global leader in the mRNA drug market.
China - It is the second-largest market with a value near USD 430 million in 2024. Its rapid growth is driven by substantial government investments in biotechnology, expanding clinical trial activities, and improving healthcare infrastructure. Additionally, the increasing number of domestic biotech startups focused on mRNA drug development further fuels the country’s market expansion.
The global mRNA drug market is characterized by intense competition among key players focusing on innovation, product pipeline expansion, and strategic collaborations. Companies are investing heavily in research and development to enhance mRNA stability, delivery methods, and therapeutic applications. The market is dominated by firms with robust portfolios of mRNA vaccines and therapeutics, particularly in oncology and infectious diseases. Leading players are also focusing on scaling up manufacturing capabilities to meet growing global demand. Strategic partnerships between biotech startups and large pharmaceutical companies are common, accelerating product development and commercialization. Competitive pricing, patent portfolios, and geographic expansion also influence market positioning. The entry of new players specializing in self-amplifying mRNA and novel lipid nanoparticle carriers is further intensifying competition, driving continuous technological advancements in the field.
Moderna, Inc.
BioNTech SE
CureVac AG
Translate Bio, Inc.
Arcturus Therapeutics Holdings Inc.
GSK plc
Sanofi S.A.
Pfizer Inc.
Translate Bio, Inc.
Acuitas Therapeutics, Inc.
The mRNA drug market leverages cutting-edge technologies that enhance drug delivery, stability, and efficacy. Lipid nanoparticle (LNP) technology remains the cornerstone for delivering mRNA molecules safely into target cells by protecting the fragile mRNA from degradation and facilitating cellular uptake. Recent advancements include optimized LNP formulations that increase mRNA stability, reduce immune reactions, and improve protein translation efficiency. Self-amplifying mRNA technology is gaining traction for its ability to produce greater amounts of protein with lower doses, enhancing treatment efficacy while reducing costs. AI and machine learning algorithms are increasingly integrated into mRNA sequence design, predicting optimal sequences for therapeutic targets and accelerating vaccine development. Additionally, scalable manufacturing platforms with automated synthesis and purification processes are being developed to meet growing global demand efficiently. These technological innovations collectively drive the expansion and diversification of mRNA therapies.
In January 2024, BioNTech announced the initiation of a Phase 3 clinical trial for its personalized mRNA cancer vaccine targeting non-small cell lung cancer, aiming to improve patient-specific immune responses.
In March 2024, Moderna expanded its manufacturing capacity by inaugurating a new state-of-the-art mRNA production facility in Europe, expected to significantly increase vaccine supply capabilities.
In August 2023, CureVac secured regulatory approval in several countries for its mRNA-based seasonal influenza vaccine, marking a key milestone for mRNA vaccines beyond COVID-19.
In November 2023, Pfizer collaborated with a leading AI company to develop accelerated mRNA vaccine design platforms, reducing the time from sequence identification to clinical testing substantially.
The mRNA drug market report covers a comprehensive analysis of market dynamics, including segmentation by type, application, and end-user across global regions. It provides detailed insights into technological advancements, competitive landscape, and regulatory frameworks shaping the industry. The report highlights key growth drivers such as increasing investments in mRNA R&D, expanding therapeutic applications, and growing demand for personalized medicine. Challenges including manufacturing complexities and regulatory hurdles are discussed with potential mitigation strategies. Regional market performance is analyzed with emphasis on emerging economies and established biotech hubs. Furthermore, the report evaluates recent developments, strategic partnerships, and pipeline products shaping future market trajectories. This extensive coverage equips stakeholders with actionable intelligence to make informed decisions and capitalize on emerging opportunities in the evolving mRNA drug landscape.
| Report Attribute / Metric | Report Details |
|---|---|
| Market Name | Global mRNA Drug Market |
| Market Revenue (2024) | USD 1,968.0 Million |
| Market Revenue (2032) | USD 3,869.9 Million |
| CAGR (2025–2032) | 8.8% |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User
|
| Key Report Deliverables | Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape, Technological Insights, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Moderna, Inc., BioNTech SE, CureVac AG, Translate Bio, Inc., Arcturus Therapeutics Holdings Inc., GSK plc, Sanofi S.A., Pfizer Inc., Translate Bio, Inc., Acuitas Therapeutics, Inc. |
| Customization & Pricing | Available on Request (10% Customization is Free) |
