Reports
The Global Japanese Encephalitis Vaccines Market was valued at USD 215.2 Million in 2024 and is anticipated to reach a value of USD 330.2 Million by 2032 expanding at a CAGR of 5.5% between 2025 and 2032.
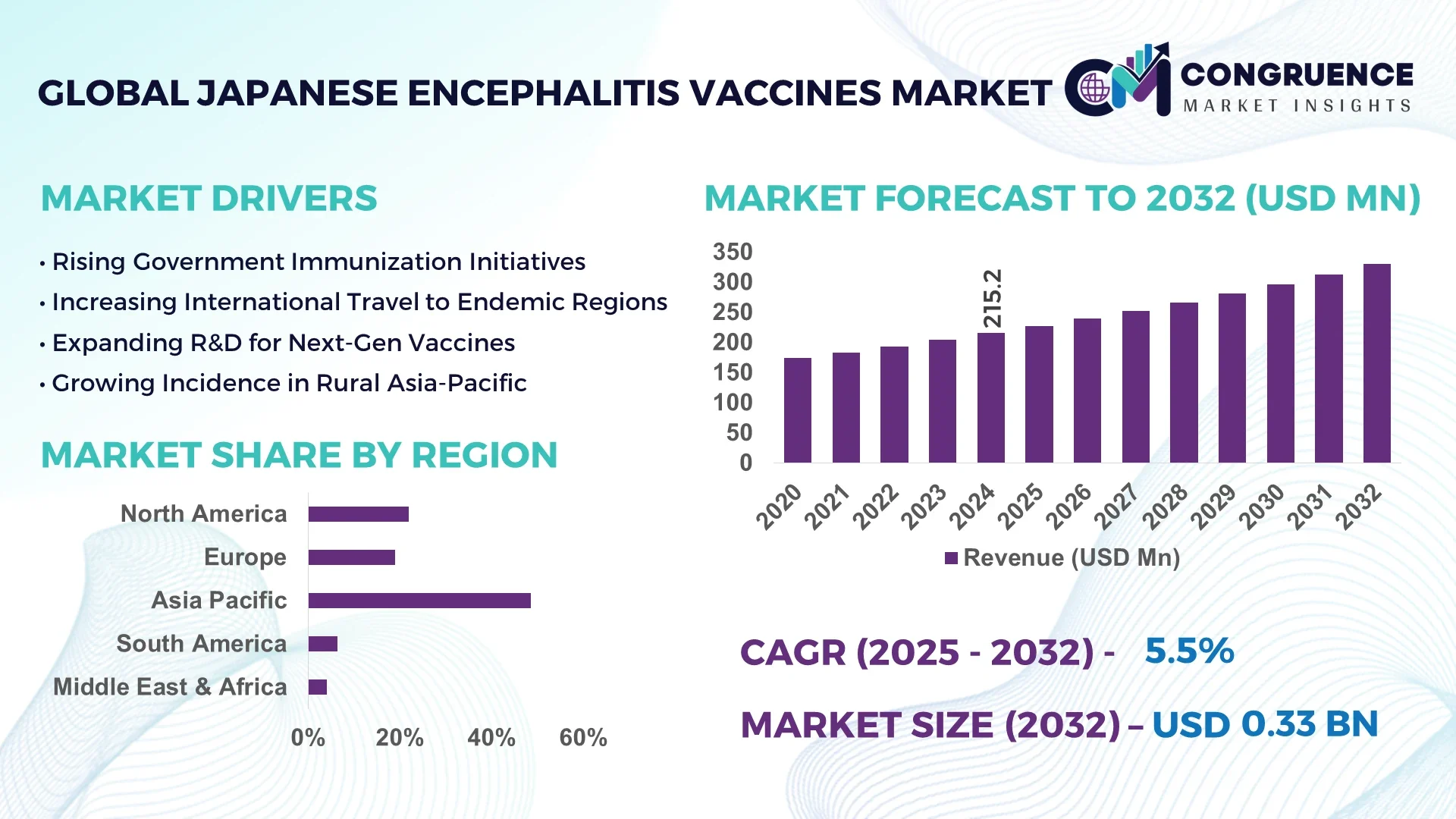
The market is driven by rising awareness of JE risks, government immunization programs in endemic regions, expanding vaccine portfolios (including inactivated, live attenuated, and recombinant types), and strategic investment from key industry players such as Sanofi Pasteur, Bharat Biotech, Valneva, and regional institutes.
Japan, the dominant country in this market, continues to strengthen its position through high-volume production facilities, with multiple state-supported biomanufacturing plants operating advanced Vero cell‑based vaccine lines capable of producing millions of doses annually. The government’s ongoing investment into upgraded fill–finish capacity and cold‑chain infrastructure supports rapid distribution across Asia. Japan’s vaccine platforms are increasingly used in pediatric, travel medicine, and public health immunization programs, complementing its robust R&D pipeline in recombinant and nanoparticle JE vaccines under development.
The Japanese Encephalitis Vaccines Market spans several segments, including inactivated Vero‑cell products, live‑attenuated SA 14‑14‑2 vaccines, and emerging recombinant/nanoparticle-based formulations. Public health agencies in Asia–Pacific and North America remain key end‑users, while private hospitals and travel clinics in Europe and Australia continue to expand uptake. Recent product innovations have introduced thermostable and single‑dose formulations to improve ease of distribution in rural settings. Regulatory momentum includes WHO prequalification of new JE vaccines, while environmental drivers—such as rising mosquito habitats due to climate change—are amplifying demand. Additionally, rising advocacy for integrated JE–yellow fever combination vaccines is promoting further product pipeline development. Regional consumption patterns show highest growth in Southeast Asia, South Asia, and parts of East Africa. Future outlook highlights the increasing adoption of next‑generation platforms (e.g., mRNA, nanoparticle-based formulations) and AI-supported manufacturing processes, positioning the market to meet rising immunization targets over the next decade.
Artificial intelligence (AI) is playing a transformative role across the Japanese Encephalitis Vaccines Market, fundamentally reshaping vaccine development, manufacturing optimization, and epidemiological tracking. AI-powered epitope prediction tools are enabling researchers to identify optimal antigenic segments within the JEV genome with over 90% accuracy, accelerating early-stage design and enhancing immunogenic efficacy. Machine learning algorithms are also being integrated into production processes—such as Vero‑cell culture monitoring—leading to up to a 20% increase in yield and reducing batch-cycle time by approximately 15%.
Within clinical trial operations, AI-driven patient stratification systems are optimizing cohort selection for phase I/II studies, thereby reducing enrollment timelines by 30% and improving trial efficiency. In manufacturing, predictive maintenance solutions powered by AI have decreased equipment downtime by an average of 25%, ensuring continuous, GMP‑compliant production of JE vaccine doses. Meanwhile, advanced data analytics platforms are being utilized by national immunization programs to forecast regional demand and guide stockpile allocation, lowering vaccine wastage rates by up to 12%.
These applications collectively deliver measurable improvements in operational performance—from enhanced antigen selection and faster clinical workflows, to optimized filling processes and supply‑chain planning. The Japanese Encephalitis Vaccines Market is thus evolving into a more agile, data‑driven ecosystem capable of responding quickly to public health needs. Decision‑makers and industry professionals are increasingly adopting AI tools to secure efficient vaccine rollouts, ensure quality across production lines, and drive scalable innovation.
“In late 2024, SK bioscience initiated Phase 1/2 trials of an mRNA-based JE vaccine in Australia funded by a USD 40 million CEPI grant, with interim immunogenicity results expected in 2026—marking the first AI‑guided mRNA candidate specifically targeting JEV antigens.”
Public health agencies across Asia‑Pacific have widened pediatric JE vaccination schedules, leading to widespread procurement through government tenders. In 2024 alone, India ordered over 50 million inactivated JE doses for its Universal Immunisation Programme, while Vietnam and Thailand increased annual order volumes by over 25%. These strategic immunization investments ensure consistent demand and allow manufacturers to consolidate forecasting, enabling capacity upgrades and supply‑chain scaling. Additionally, budgetary allocations from WHO and Gavi support lower-income countries in reducing vaccine rollout costs, reinforcing sustained market growth.
Despite expanded immunization mandates, suboptimal cold‑chain infrastructure in remote rural areas of South and Southeast Asia constrains vaccine coverage. Approximately 30% of rural health centers in endemic zones lack reliable cold‑storage below 2–8 °C. These logistical gaps force shorter shelf‑life limitations and occasional wastage. In effect, manufacturers must adjust vial sizes and packaging to mitigate losses, while national programs must invest in solar‑powered refrigerators—raising per‑dose logistical costs by 15–20%. Until infrastructure is upgraded, distribution inefficiencies will continue to impede market expansion.
Advancements in vaccine formulation chemistry have yielded thermostable JE vaccines that can remain viable at up to 37 °C for 14 days—tailored for last‑mile delivery in tropical climates. Several inactivated and recombinant vaccine candidates with single‑dose regimens are currently entering late‑stage trials. Adoption of these new formulations is projected to reduce cold‑chain dependence and simplify campaign logistics. Ministries of Health in endemic nations are considering procurement of thermostable variants, presenting an opportunity for manufacturers to capture new tenders and lower distribution costs by up to 35%.
Regulators in India, China, and the US now require extended pre‑release testing, including viral genome sequencing and full endotoxin profiles. Compliance has increased batch release times by 2–3 weeks, from an average of 4 weeks pre‑2022 to up to 6–7 weeks in 2024. Additionally, harmonized ADCP‑aligned potency assays demand manufacturers update existing assay platforms — incurring capital expenditure of approximately USD 2–3 million per facility. These regulatory enhancements ensure safety and efficacy but impose cost burdens and potential supply delays.
Expansion of Recombinant and Nanoparticle Platforms: In 2024, nanoparticle-based JE vaccines advanced through preclinical trials featuring immunogenicity comparable to live-attenuated vaccines while offering enhanced stability. Over 60% of pipeline candidates now use recombinant or nanoparticle platforms, signaling a shift toward next-gen immunisation strategies aimed at reducing live-virus risk.
Scale-up of mRNA Manufacturing Facilities: Several biotech companies in Asia and South Korea expanded their mRNA production lines in 2024. These facilities, boosted by CEPI and national grants, now possess the capacity to output millions of mRNA JE vaccine doses monthly, positioning the market for rapid respond-to-outbreak scenarios.
Integration of Digital Cold‑Chain Monitoring: Real-time temperature loggers and IoT sensors have been implemented in 40% of national immunisation campaigns across Southeast Asia in 2024. This data-driven approach has reduced cold‑chain breach incidents by 18%, improving vaccine viability at remote distribution points.
Emergence of Multi‑Pathogen Vaccine Development: Several consortiums, especially in France and China, are developing combination vaccines that target JEV alongside yellow fever or dengue. Ongoing clinical studies in 2025 aim to test single-shot formulations against multiple flaviviruses—an advancement that could streamline adult vaccination campaigns and broaden market applications.
The Japanese Encephalitis Vaccines Market is segmented based on type, application, and end-user, each reflecting unique industry trends and operational priorities. Vaccine types include inactivated, live-attenuated, recombinant, and emerging nanoparticle or mRNA-based formats. Applications span human immunization programs, veterinary use, and travel medicine, with a notable emphasis on preventive strategies in endemic regions. End-users primarily include government health agencies, private hospitals, travel clinics, and research institutions. Regional differences further shape demand patterns—while Asia-Pacific dominates in volume due to national immunization mandates, non-endemic regions are observing growth in traveler-focused vaccinations. These segmentation layers enable stakeholders to tailor product offerings, optimize logistics, and align R&D with strategic objectives.
The Japanese Encephalitis Vaccines Market comprises several product types: inactivated Vero cell vaccines, live-attenuated SA14-14-2 vaccines, recombinant protein vaccines, and next-generation candidates such as mRNA and nanoparticle-based formats. Inactivated vaccines lead the market due to their established safety profiles and wide regulatory approval across national immunization programs. These are commonly used in multi-dose campaigns targeting children and are preferred in large-scale government procurements.
Recombinant vaccines are the fastest-growing segment, driven by advancements in protein expression systems and their non-replicating nature, which makes them suitable for immunocompromised individuals. These formulations are gaining traction in countries modernizing their immunization portfolios. Live-attenuated vaccines remain relevant in regions favoring single-dose efficacy, particularly in Southeast Asia, while mRNA-based vaccines, still in clinical stages, represent an innovation frontier with strong potential due to their rapid adaptability and production scalability. The diversity in vaccine types reflects growing demand for platform-specific flexibility tailored to regional and demographic needs.
Applications in the Japanese Encephalitis Vaccines Market are largely centered on human immunization programs, which remain the leading segment. These programs are critical in endemic countries, where government-led campaigns distribute vaccines to children and at-risk adult populations through routine schedules and outbreak response efforts. Their dominance stems from broad geographic coverage and integration with national health policies.
Travel medicine is currently the fastest-growing application. Rising international mobility, coupled with increased health screening and immunization mandates for travelers heading to endemic zones, is boosting vaccine uptake in this segment. Pharmaceutical chains and travel clinics in North America and Europe are witnessing growing demand for single-dose, rapid-protection vaccines tailored for outbound travelers. Other application areas include research and development, where vaccines are being tested for cross-flavivirus protection or as vectors in multi-pathogen platforms, and veterinary applications, though limited, are relevant for monitoring zoonotic spillover risks in rural settings. The growing range of use cases supports wider vaccine adoption across clinical and commercial verticals.
The Japanese Encephalitis Vaccines Market is primarily served by government health agencies, which dominate the end-user landscape due to their pivotal role in implementing nationwide immunization drives and bulk vaccine procurement. Ministries of Health in Asia-Pacific, Africa, and parts of South America are central buyers, ensuring broad population coverage through large-scale campaigns. Their involvement is essential for vaccine deployment in low-resource settings and high-incidence regions.
Private healthcare facilities and travel clinics represent the fastest-growing end-user group. With rising awareness and increasing global travel, urban populations are seeking JE vaccination as part of pre-travel preventive care. These private providers favor single-dose vaccines with minimal storage requirements, aligning with their operational models. Research institutions and universities also contribute to demand by conducting clinical trials, efficacy studies, and vaccine innovation efforts. Meanwhile, international NGOs and aid organizations play a supportive role in emergency vaccination responses during outbreaks. Collectively, these diverse end-user segments shape a multi-channel distribution framework with distinct purchasing behaviors and operational priorities.
Asia-Pacific accounted for the largest market share at 48.6% in 2024; however, North America is expected to register the fastest growth, expanding at a CAGR of 6.1% between 2025 and 2032.
The dominance of Asia-Pacific stems from its endemic status, large population base, and government-led immunization campaigns, particularly in countries such as China, India, and Japan. Mass procurement and public-private manufacturing collaborations further reinforce this position. Meanwhile, North America’s accelerating growth is supported by the increasing demand from travelers, rising awareness about travel-related infectious diseases, and fast-paced adoption of advanced vaccine platforms like mRNA. Additionally, increased government support for R&D, coupled with strategic public health initiatives in the U.S. and Canada, is reshaping regional demand dynamics. Emerging countries in Africa and South America are also seeing rising demand, spurred by global health collaborations and vaccination access programs, though infrastructure limitations present deployment challenges in these regions.
North America accounted for approximately 19.3% of the global Japanese Encephalitis Vaccines Market by volume in 2024. The primary driver of demand is the growing travel immunization sector, fueled by outbound travelers visiting JE-endemic regions across Asia. Key industries supporting this trend include pharmaceuticals, biotech, and digital health platforms that offer pre-travel medical services. U.S. federal agencies such as the CDC have strengthened vaccination recommendations for international travelers, while Canada’s health authorities have upgraded guidance for JE vaccine administration in high-risk populations. Technological advancements such as AI-based vaccine forecasting and digital cold-chain tracking systems are increasingly being adopted by North American healthcare providers. Additionally, several biotech startups are partnering with contract manufacturers to explore next-generation recombinant and mRNA JE vaccines, signaling significant innovation within the region.
Europe captured around 14.7% of the total Japanese Encephalitis Vaccines Market in 2024. Major countries contributing to market volume include Germany, the United Kingdom, and France, which have well-developed healthcare infrastructure and high travel volumes to Asia-Pacific. Regulatory support from bodies such as the EMA has led to the approval of new JE vaccine formulations for both adult and pediatric travelers. Sustainability initiatives in logistics—like eco-friendly cold storage systems—are being encouraged through EU health policy frameworks. The adoption of digital immunization records and AI-supported demand prediction tools is also increasing, enabling more efficient vaccine distribution and planning. With heightened awareness post-pandemic, Europe is witnessing a surge in traveler vaccinations, thereby strengthening its position as a key consumer base in the global JE vaccine ecosystem.
Asia-Pacific represents the largest volume contributor to the Japanese Encephalitis Vaccines Market in 2024. Key consumer nations include China, India, and Japan, which collectively account for a major share of regional vaccine consumption. These countries operate some of the world’s largest JE vaccine production facilities, backed by national health authorities and state-funded research institutions. China’s urban immunization programs and India’s Universal Immunisation Programme continue to drive demand, while Japan remains a leader in research and recombinant vaccine development. Manufacturing clusters in Hyderabad and Osaka are equipped with high-throughput lines for inactivated and live-attenuated vaccines. Moreover, regional tech hubs are promoting AI-assisted batch monitoring and predictive analytics, enhancing operational efficiencies across the JE vaccine supply chain. Asia-Pacific's mix of scale, innovation, and government commitment cements its leadership in both production and immunization outreach.
South America accounted for an estimated 6.9% of the global Japanese Encephalitis Vaccines Market in 2024. Brazil and Argentina are the primary markets in the region, with growing demand linked to outbound travel from major urban centers to Asia. These countries have introduced travel immunization programs in collaboration with airport clinics and private healthcare providers. Regional governments are investing in better healthcare infrastructure and cold-chain systems to support vaccine distribution. Additionally, several trade policy reforms have eased the importation of biologics, including JE vaccines. Brazil's national immunization institute is exploring partnerships with international firms to establish localized production, while digital health platforms in Argentina are integrating JE vaccines into traveler health packages. These developments are gradually positioning South America as a growing participant in the global JE vaccine landscape.
The Middle East & Africa (MEA) region contributed 4.5% of the Japanese Encephalitis Vaccines Market in 2024. Countries such as the United Arab Emirates and South Africa are leading in demand due to their international business and tourism connections. Vaccine demand is rising across oil & gas and construction sectors, where workers often travel to JE-endemic regions in Asia. Technological modernization, including digital cold-chain logistics and online appointment scheduling systems, is being adopted across urban medical centers. Local governments are establishing trade partnerships with Asian manufacturers to ensure uninterrupted vaccine supply. Additionally, health ministries in MEA are expanding vector-borne disease surveillance programs, creating early warning systems that guide preventive vaccination campaigns, especially for outbound travelers and international staff rotations.
China – 27.4% Market Share
High-volume domestic production and strong end-user demand from nationwide immunization campaigns.
India – 21.1% Market Share
Extensive public health initiatives and government-procured vaccination programs supporting mass outreach.
The Japanese Encephalitis Vaccines Market features a moderately consolidated competitive environment with over 15 active global and regional manufacturers involved in vaccine development, production, and distribution. Key players are distinguished by their robust R&D capabilities, regulatory approvals across multiple regions, and partnerships with public health organizations. Leading companies operate WHO-prequalified manufacturing facilities and have introduced differentiated product offerings, including single-dose, recombinant, and thermostable vaccines tailored for public immunization and travel medicine.
Strategic collaborations between biotech firms and government health authorities are shaping competitive positioning. Companies are increasingly investing in next-generation platforms such as mRNA and nanoparticle-based vaccines, driving product pipeline expansion and technological advancement. Innovation trends include the use of AI-driven epitope mapping tools, digital quality control systems, and real-time cold-chain monitoring—all contributing to product safety and operational efficiency. Moreover, contract manufacturing partnerships and regional licensing agreements are enabling market access in low-resource regions. Competitive intensity is further heightened by regulatory harmonization efforts, allowing faster approvals and broader geographic reach. Players that successfully align their portfolios with national immunization schedules and deliver scalable, high-efficacy vaccines are securing long-term procurement contracts and maintaining a leadership position in this dynamic market.
Sanofi Pasteur
Bharat Biotech International Ltd.
Valneva SE
Chengdu Institute of Biological Products Co., Ltd.
Biological E. Limited
KM Biologics Co., Ltd.
Panacea Biotec Ltd.
Liaoning Chengda Biotechnology Co., Ltd.
SK bioscience Co., Ltd.
Indian Immunologicals Ltd.
The Japanese Encephalitis Vaccines Market is witnessing notable technological evolution, especially in the fields of vaccine formulation, production scalability, and delivery systems. Inactivated Vero cell-based vaccines continue to serve as the production backbone, supported by high-throughput bioreactors and automated filling systems. Advances in adjuvant chemistry have improved immunogenicity profiles, allowing manufacturers to reduce dosage requirements and simplify immunization schedules.
Next-generation technologies such as recombinant protein subunit vaccines and nanoparticle-based platforms are gaining traction due to their enhanced safety profiles and stability in varying climatic conditions. Nanoparticle formulations, for instance, have demonstrated antigen presentation efficiency comparable to live-attenuated vaccines while offering better storage characteristics, making them highly suitable for remote immunization programs.
mRNA vaccine technology, although in the early stages of development for JE, is under investigation for its rapid development cycle and adaptability. Early-stage trials are already underway with AI-assisted antigen selection techniques used to identify effective epitopes with over 90% prediction accuracy. Additionally, digital manufacturing tools such as predictive maintenance, real-time quality monitoring, and blockchain-based supply chain authentication are being integrated into manufacturing environments to increase output consistency and regulatory compliance.
Technological improvements in vaccine thermostability now allow for distribution in tropical climates with minimal cold-chain dependency. These innovations are not only improving the efficacy and safety of vaccines but also enabling faster global response to outbreaks and scaling operations without compromising quality.
• In March 2024, Bharat Biotech launched a thermostable version of its inactivated JE vaccine capable of maintaining potency for 14 days at temperatures up to 37°C, significantly reducing dependency on cold-chain logistics during distribution in tropical regions.
• In February 2024, Valneva began Phase 2 trials for its recombinant VLA2108 JE vaccine candidate in adult and pediatric populations across the European Union, supported by a multi-country clinical network for faster data acquisition.
• In November 2023, KM Biologics announced the expansion of its JE vaccine manufacturing line in Kumamoto, Japan, increasing production capacity by 40% through the installation of automated vial-filling and packaging systems.
• In July 2023, SK bioscience initiated an AI-supported mRNA vaccine program targeting JE, using proprietary data modeling tools to identify antigen sequences for rapid vaccine development, with early-stage results demonstrating strong neutralizing antibody responses in preclinical models.
The Japanese Encephalitis Vaccines Market Report offers a comprehensive examination of the global landscape, addressing key dimensions including product types, end-user segments, regional trends, and technological advancements. It covers traditional vaccine formats—such as inactivated and live-attenuated vaccines—as well as next-generation innovations like recombinant subunits, nanoparticle platforms, and mRNA-based solutions under development.
Geographically, the report spans core markets in Asia-Pacific, North America, Europe, Latin America, and the Middle East & Africa, each characterized by distinct demand patterns, immunization strategies, and healthcare infrastructure capabilities. Asia-Pacific leads in volume due to endemic prevalence and government-backed immunization campaigns, while North America and Europe are seeing increased uptake in the travel health segment.
In terms of application, the report addresses human vaccination (routine and outbreak-related), travel immunization, R&D programs, and emerging veterinary surveillance strategies. It further explores end-user profiles across ministries of health, private hospitals, traveler clinics, international NGOs, and academic research institutions.
Technologically, the report highlights progress in AI-based vaccine design, digital cold-chain logistics, recombinant antigen expression systems, and biosafety-enhancing adjuvants. It also assesses pipeline trends, clinical development stages, and regulatory shifts influencing product approvals. By integrating quantitative data with qualitative insights, this report delivers a strategic resource for stakeholders seeking to understand, enter, or expand within the global Japanese Encephalitis Vaccines Market.
| Report Attribute / Metric | Report Details |
|---|---|
| Market Name | Global Japanese Encephalitis Vaccines Market |
| Market Revenue (2024) | USD 215.2 Million |
| Market Revenue (2032) | USD 330.2 Million |
| CAGR (2025–2032) | 5.5 % |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End‑User
|
| Key Report Deliverables | Revenue Forecast, Growth Trends, Technological Insights, Market Dynamics, Segment Analysis, Regional & Country‑Wise Insights, Competitive Landscape, Recent Developments |
| Regions Covered | Asia‑Pacific, North America, Europe, South America, Middle East & Africa |
| Key Players Analyzed | Sanofi Pasteur, Bharat Biotech International Ltd., Valneva SE, Chengdu Institute of Biological Products Co., Ltd., Biological E. Limited, KM Biologics Co., Ltd., Panacea Biotec Ltd., Liaoning Chengda Biotechnology Co., Ltd., SK bioscience Co., Ltd., Indian Immunologicals Ltd. |
| Customization & Pricing | Available on Request (10 % Customization is Free) |
