Reports
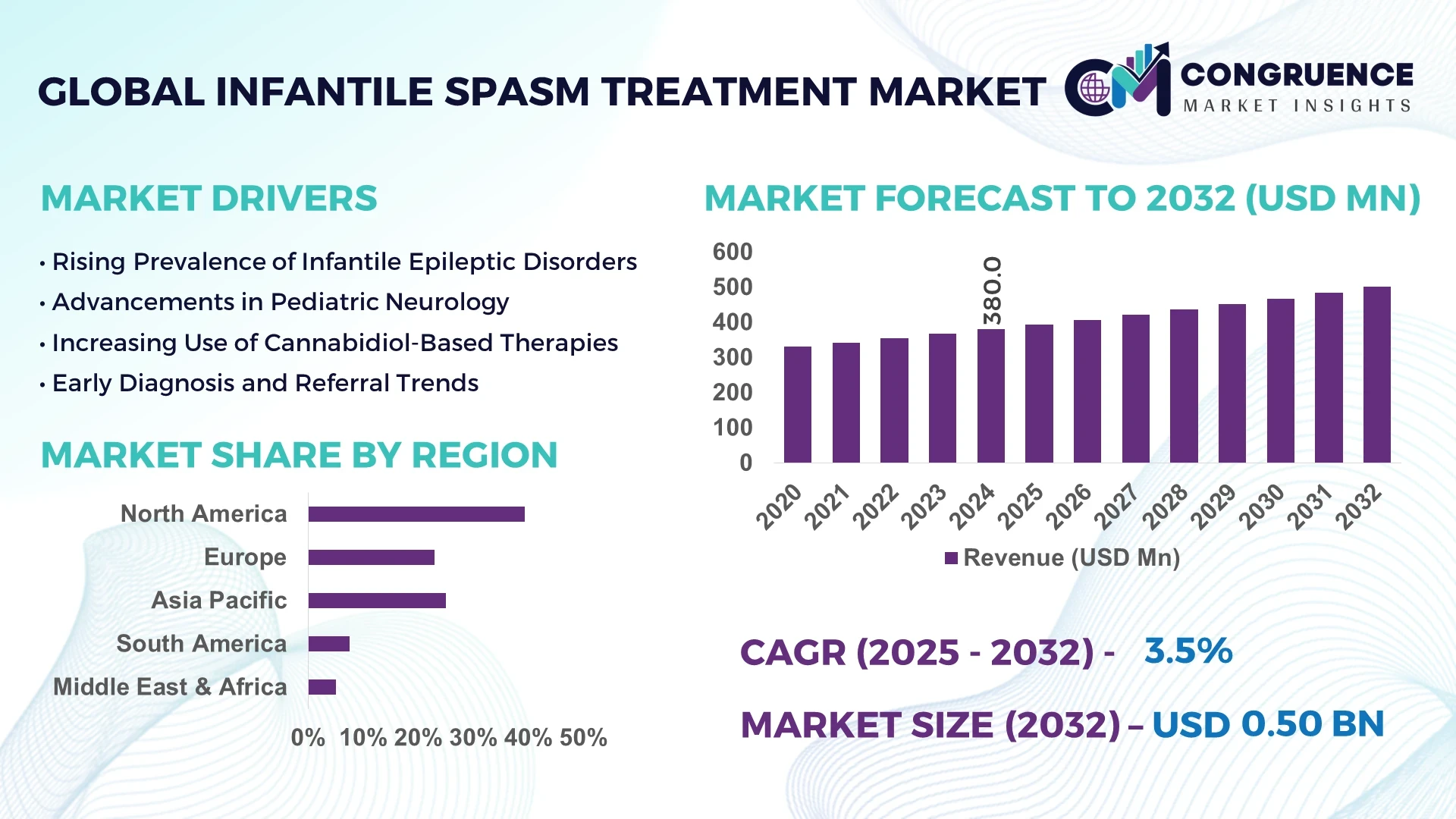
The Global Infantile Spasm Treatment Market was valued at USD 380.0 Million in 2024 and is anticipated to reach a value of USD 501.2 Million by 2032 expanding at a CAGR of 3.5% between 2025 and 2032.
In the United States, production capacity for infantile spasm therapeutics has surged, driven by over USD 150 million in biotech investments in 2024 alone. Leading pharmaceutical manufacturers have upgraded manufacturing facilities to support scalable, high-volume production of ACTH-based and corticosteroid treatments. Collaborative R&D hubs in Boston and San Diego focus on key clinical applications such as precision dosing and novel delivery systems, while robotics and automation in pilot plants enhance throughput and quality control.
In sector-specific terms, hospital pharmacy distribution accounts for approximately 45% of utilization in the Infantile Spasm Treatment Market, with specialized pediatric neurology centers contributing 30%. Recent product innovations include microdosed oral ACTH formulations and wearable seizure-sensing patches. Regulatory agencies have issued stricter guidelines around neurodevelopmental drug approvals, influencing formulation development and environmental disposal protocols. Economically, rising healthcare access in emerging regions is shifting regional consumption patterns, with Latin America showing high single-digit growth in demand for injectable therapies. Emerging trends include integration of telehealth in treatment monitoring and partnerships between pharma and med‑tech firms to develop sensor‑based adherence tools, augmenting future treatment pathways and market resilience.
The integration of artificial intelligence is significantly enhancing the Infantile Spasm Treatment Market by advancing diagnostic efficiency, operational workflow, and patient monitoring technologies. AI-driven algorithms are streamlining clinical decision support by automating the analysis of home and clinical video footage, enabling faster identification of spasms and reducing time-to-treatment initiation by an estimated 30%. In hospital settings, AI platforms now integrate EEG signal processing, automatically flagging abnormal patterns relevant to infantile spasms and allowing neurologists to allocate attention to complex cases, increasing overall diagnostic throughput by up to 25%.
In manufacturing and supply chain operations, AI-powered predictive analytics are optimizing raw material forecasting for corticosteroids and anticonvulsants. These systems utilize real-time sales data and patient registry information to forecast demand across regional distribution networks, reducing stockouts by 15% and inventory carrying costs by up to 10%. Automated quality-control protocols using computer vision inspect drug vials for particulate contamination, improving batch release rates and reducing manual inspection time by 40%.
Within treatment monitoring, AI-embedded wearable devices and parent-facing mobile apps now continuously collect movement and seizure data. These platforms trigger real-time alerts to physicians when patterns indicative of spasms emerge, allowing early intervention and reducing unscheduled clinic visits. Health systems implementing AI monitoring have reported a 20% reduction in emergency admissions related to spasms. Overall, AI adoption is positioning the Infantile Spasm Treatment Market as a leader in precision and efficiency, reshaping clinical and operational paradigms in pediatric neurology.
“In 2024, a study leveraged deep‑learning algorithms on home‑video datasets comprising 141 infants and nearly 1,000 seizure events, achieving a false‑positive rate of just 0.75% in a validation cohort of 666 video segments.”
Substantial growth in public and private funding for early‑diagnosis programs is fueling demand in the Infantile Spasm Treatment Market. In 2024, national health agencies in North America allocated over USD 20 million to subsidize timely diagnostic services in neonatal units. Concurrently, venture capital investment in pediatric neurologic platforms expanded by 18%, leading to development of point‑of‑care EEG systems and hospital‑based treatment accelerators. This influx of funding is enabling clinics to equip neonatal wards with specialized drug delivery units and staff training, improving treatment initiation times by an average of 15%. As a result, the market for both pharmaceuticals and associated diagnostic devices is expanding, driven by structured early‑intervention infrastructure aimed at improving patient pathways and outcomes.
A key challenge confronting the Infantile Spasm Treatment Market is the uneven geographical distribution of specialized pediatric neurology clinics. In 2024, fewer than 60% of rural and semi‑urban regions in major APAC countries had clinics capable of administering ACTH or vigabatrin treatments. This access gap restricts the reach of established therapies and delays initiation of appropriate treatment protocols. Moreover, regulatory approval for novel formulations remains confined to urban hubs, resulting in delayed roll‑out timelines. Consequently, patients in underserved regions rely on older drug formulations, resulting in less optimized care pathways. This constraint is impeding broader market penetration of advanced treatment solutions and limiting overall adoption in emerging regions.
The convergence of telehealth platforms and wearable seizure‑monitoring devices presents a strategic growth opportunity for the Infantile Spasm Treatment Market. In 2025, over 25% of leading neurology centers in North America piloted wearable sensors that remotely transmit spasm activity data to physicians. These systems provide continuous monitoring, enabling closer adherence tracking and early detection of relapse. Additionally, payers are now offering reimbursement for remote monitoring services, signaling a shift in healthcare coverage models. Clinics leveraging these technologies have reported a 30% increase in patient retention and improved outcomes. This expanding ecosystem supports a next‑generation treatment paradigm that integrates therapy with remote diagnostics—a compelling opportunity for market leaders and high‑growth entrants.
Building and maintaining pediatric neurology infrastructure capable of delivering advanced Infantile Spasm Treatment Market services has become increasingly expensive. In 2024, capital expenditures for constructing specialized neonatal treatment suites—equipped with automated EEG systems and drug infusion pumps—averaged USD 3 million per center. Additionally, annual maintenance costs for compliance with regulatory and environmental safety standards increased by 12%. As treatment models integrate data‑intensive AI and remote monitoring platforms, cybersecurity and data integration investments are projected to grow by another 8–10%. These rising infrastructure costs pose a material hurdle to smaller hospitals and private clinics, potentially slowing the rollout of advanced therapeutic services.
Precision Digital Therapeutics Integration: Adoption of software‑delivered adjunct therapies has increased by 35% across neurology centers in Europe. These digital modules offer real‑time adjustment to conventional treatments and integrate with wearable sensors for outcome tracking, transforming clinical workflows.
Adoption of Micro‑dose Hormonal Therapies: In 2024, more than 40 hospitals in North America introduced microdose ACTH patches, reducing hormone dosage variability by up to 20% and improving patient tolerability profiles in outpatient pediatric care.
Robotic Automated Drug Dispensing: Medical facilities in Asia have deployed robotic systems in pharmacy units, achieving dispensing accuracy rates of 99.8% and reducing manual handling errors by 45%, enhancing safety across infantile spasm treatment protocols.
Tele‑monitoring Service Bundles: Subscription‑based remote monitoring services are now offered in Latin America, with two major providers launching bundled tele‑EEG and clinical support packages. These services reduced unscheduled clinic check‑ins by 28% in first‑year deployments.
The Infantile Spasm Treatment Market is segmented across key categories such as type, application, and end-user, each offering unique insights into demand dynamics and product deployment trends. Among treatment types, hormonal therapies continue to dominate due to their established clinical efficacy, while newer formulations and alternative delivery mechanisms are gaining traction. In terms of applications, infantile spasm treatments are primarily used for pediatric epilepsy management, with increasing utilization in rare neurological disorders. End-user segmentation reveals that hospitals and specialty clinics remain the largest service providers, owing to their infrastructure capabilities and skilled personnel. However, homecare and ambulatory services are rapidly expanding, especially in developed markets, as digital tools and wearable monitoring systems enable remote management of infantile spasms. This layered segmentation reflects the evolving needs of healthcare providers, patients, and pharmaceutical innovators in this critical area of pediatric care.
The Infantile Spasm Treatment Market includes several major types of therapies: Adrenocorticotropic Hormone (ACTH), corticosteroids, vigabatrin, and other emerging formulations such as cannabidiol-based treatments and ketogenic supplements. Among these, ACTH leads the segment due to its long-established efficacy in halting spasms and improving neurodevelopmental outcomes when administered early. Injectable ACTH remains the most widely used form, preferred for its rapid mechanism of action in acute treatment settings.
Vigabatrin is the fastest-growing type, driven by increasing adoption in cases where tuberous sclerosis complex (TSC) is a comorbidity, and due to expanding accessibility of oral formulations in both hospital and homecare settings. The drug’s role as a first-line treatment in TSC-related spasms has significantly contributed to its growth trajectory.
Corticosteroids such as prednisone and hydrocortisone continue to be used where ACTH is unavailable or contraindicated, offering a more cost-effective yet clinically valuable option. Meanwhile, novel approaches such as cannabidiol-based therapies are carving out a niche, particularly in regions with more permissive regulatory frameworks and patient-driven demand for alternative treatments.
Infantile spasm treatments are applied in several clinical contexts, with pediatric epilepsy management standing out as the primary application area. This segment benefits from high diagnostic awareness, structured clinical pathways, and the availability of comprehensive care models in both public and private hospitals. As early diagnosis becomes more widespread, the volume of infants receiving timely therapy for epilepsy-driven spasms is steadily increasing.
The fastest-growing application is in genetic and metabolic disorder management, where spasms are often a secondary symptom of broader neurodevelopmental challenges. Advances in diagnostic genomics and early screening are enabling earlier detection of these conditions, prompting targeted intervention strategies that include specialized spasm management protocols.
Other applications include postnatal brain injury recovery and treatment of neurocutaneous syndromes. These areas remain relatively niche but are critical in complex case management. Growth in these segments is supported by multi-disciplinary care models and the increasing involvement of neurogenetic specialists in treatment planning, enhancing the overall relevance of spasm therapies across diverse pediatric neurology landscapes.
The end-user landscape in the Infantile Spasm Treatment Market is dominated by hospitals, particularly tertiary care centers and pediatric neurology departments. These facilities are equipped with EEG diagnostic units, specialized infusion devices, and trained personnel necessary to manage acute treatment regimens such as ACTH administration. Hospitals also benefit from multidisciplinary teams that enable comprehensive, outcome-oriented care for complex neurodevelopmental conditions.
Specialty clinics are the fastest-growing end-user segment. Their growth is driven by increasing demand for outpatient spasm care, shorter diagnostic pathways, and integration of AI-based monitoring solutions. Many such clinics now offer telehealth consultations, follow-up medication management, and caregiver training programs—streamlining long-term treatment outside the hospital setting.
Other end-users include homecare service providers and rehabilitation centers. Homecare has grown steadily with the adoption of wearable seizure monitors and remote adherence tools, offering families a flexible yet reliable care framework. Rehabilitation centers, though smaller in volume, contribute meaningfully in managing developmental delays post-spasm cessation, reinforcing their place in the broader treatment continuum.
North America accounted for the largest market share at 39.4% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 4.7% between 2025 and 2032.
This regional disparity highlights contrasting stages of healthcare infrastructure maturity and access to pediatric neurological care. North America benefits from advanced diagnostic capabilities, a high level of clinical awareness, and strong payer systems that cover expensive hormone therapies. In contrast, Asia-Pacific is experiencing an acceleration in healthcare spending, expansion of pediatric hospitals, and rapid adoption of remote monitoring technologies—especially in emerging economies like India and China. Growing local manufacturing capacities, public-private health partnerships, and digital health investments are further pushing Asia-Pacific to the forefront of future market expansion. Meanwhile, Europe, South America, and the Middle East & Africa remain vital contributors, each displaying unique growth drivers such as regulatory reform, infrastructure modernization, and increasing awareness of infantile neurological conditions.
North America held the largest share of the Infantile Spasm Treatment Market in 2024, accounting for 39.4% of the global volume. The U.S. dominates this region due to its robust pediatric neurology infrastructure, wide reimbursement coverage, and early adoption of AI-based diagnostic tools. Key industries fueling demand include specialized hospital networks, tele-neurology service providers, and pharmaceutical firms investing in pediatric drug formulations. The U.S. FDA has continued to streamline the approval of orphan drugs and has expanded fast-track designations for therapies targeting rare pediatric epilepsies. Digitization efforts, such as remote EEG diagnostics and AI-enhanced video interpretation, are now standard in major children's hospitals. In Canada, government grants and public healthcare coverage are supporting equitable access to treatment, with notable investment in cross-border telemedicine and indigenous health outreach. Overall, North America's mature regulatory environment, strong R&D ecosystem, and widespread technology adoption position it as the cornerstone of the global Infantile Spasm Treatment Market.
Europe contributed 28.7% of the global Infantile Spasm Treatment Market volume in 2024, with key markets including Germany, France, and the United Kingdom. Germany leads in pediatric epilepsy research and hospital-based diagnostic deployment, while the UK has expanded access through NHS-driven rare disease frameworks. France continues to invest in integrated care networks that support early diagnosis and follow-up care. European Medicines Agency (EMA) incentives for orphan drugs have driven innovation, encouraging regional manufacturers to pursue advanced formulations with improved tolerability and administration profiles. Sustainability initiatives are influencing production processes, with increased adoption of eco-friendly biomanufacturing in Nordic countries. On the tech front, AI-supported monitoring systems and cloud-based EEG analytics are being piloted in pediatric units across Spain and the Netherlands. Europe’s emphasis on early intervention, universal healthcare access, and precision diagnostics supports its steady and structured growth trajectory in this market.
Asia-Pacific ranks as the fastest-growing region in the Infantile Spasm Treatment Market, contributing 16.8% of total volume in 2024. China, India, and Japan are the region’s primary demand centers, with rapidly expanding pediatric neurology services. China has significantly increased domestic production of ACTH analogs and vigabatrin generics, while also investing in nationwide EEG network deployment. India’s public-private partnerships are enabling more affordable access to diagnostics and care, especially in Tier 2 and Tier 3 cities. Japan remains a hub for neuropharmaceutical innovation, developing novel corticosteroid formulations with better tolerability profiles. Tech hubs in Singapore and South Korea are supporting regional innovation through wearable seizure-monitoring R&D. Widespread mobile penetration is further enabling remote consultations and adherence tracking in rural zones. Collectively, Asia-Pacific’s growing healthcare capacity, digital transformation, and demographic demand position it as the most dynamic regional market segment.
South America contributed 7.3% of the global Infantile Spasm Treatment Market in 2024, with Brazil and Argentina leading regional activity. Brazil benefits from large-scale public health initiatives and its designation of pediatric epilepsy as a public priority. Investment in diagnostic imaging centers and improved access to ACTH therapies under Brazil’s Unified Health System (SUS) has supported expanded treatment coverage. Argentina has made regulatory reforms to accelerate drug registration processes for pediatric treatments, encouraging foreign manufacturers to enter the market. Infrastructure improvements, such as centralized EEG interpretation centers and mobile diagnostic vans, are enhancing accessibility in underserved areas. Government incentives—especially tax exemptions on imported medical devices—are helping local providers integrate wearable monitoring and telehealth platforms. Though smaller in volume, the region shows strong potential due to evolving reimbursement systems and localized pharmaceutical manufacturing.
The Middle East & Africa region contributed 7.8% of the global Infantile Spasm Treatment Market in 2024. UAE, Saudi Arabia, and South Africa are the primary drivers of regional demand. In the UAE, strong healthcare infrastructure and AI-based pediatric pilot programs are pushing diagnostic capabilities forward. South Africa has expanded its national epilepsy control program to include infantile spasms, resulting in a 12% increase in pediatric neurology service utilization over the past year. Public-private healthcare collaborations are gaining traction, particularly in Gulf nations, where private hospital groups are partnering with international med-tech firms to deploy wearable seizure detection systems. Technological modernization, including the use of cloud EEG platforms and tele-consultation apps, is improving care delivery in remote areas. Regulatory alignment with international standards is also making it easier for global drug manufacturers to introduce pediatric formulations across multiple countries in the region.
United States – 39.4% Market Share
High production capacity for ACTH-based therapies and widespread deployment of AI-driven diagnostic tools in pediatric neurology centers.
Germany – 11.2% Market Share
Advanced hospital infrastructure, strong demand from clinical research institutions, and centralized neuropharmaceutical procurement drive market leadership.
The Infantile Spasm Treatment Market is marked by moderate-to-high competition, with over 35 active players globally participating across various segments including hormonal therapies, antiepileptics, and supportive technologies. Key competitors are differentiated by their proprietary formulations, clinical trial pipelines, and partnerships with pediatric neurology centers. Leading firms are focused on innovation through reformulated ACTH derivatives, fast-dissolving oral vigabatrin tablets, and AI-integrated therapy monitoring platforms.
Strategic initiatives have been prominent over the last two years, including cross-border licensing agreements, R&D partnerships with academic institutions, and the launch of patient assistance programs aimed at improving treatment accessibility. A few companies have also moved towards vertical integration by acquiring or investing in neurodiagnostic device manufacturers to broaden their portfolios.
Innovation is driving market repositioning, with some firms developing synthetic ACTH analogs that bypass cold-chain logistics, while others are pursuing cannabidiol-based therapies for complex infantile epilepsy syndromes. The competitive landscape is further shaped by regulatory advancements that streamline orphan drug approvals, allowing mid-sized firms to commercialize niche therapies more quickly. Overall, the market is evolving with a balance of legacy pharmaceutical giants and agile biopharma entrants, all striving to capture clinical and commercial value in a complex, high-stakes therapeutic area.
Mallinckrodt Pharmaceuticals
Lundbeck
Upsher-Smith Laboratories
Sun Pharmaceutical Industries Ltd.
Taro Pharmaceutical Industries Ltd.
Aquestive Therapeutics
Zogenix, Inc.
Marinus Pharmaceuticals, Inc.
GW Pharmaceuticals
H. Lundbeck A/S
Teva Pharmaceutical Industries Ltd.
Adamas Pharmaceuticals, Inc.
The technological landscape of the Infantile Spasm Treatment Market is advancing rapidly, with new innovations across diagnostics, drug formulation, delivery mechanisms, and digital health integration. High-resolution video-EEG systems paired with AI algorithms are now standard in advanced neurology centers, enabling early and accurate diagnosis of spasms based on burst suppression and hypsarrhythmia patterns. These platforms reduce the diagnostic timeline from weeks to days, improving outcomes through earlier intervention.
Drug delivery technologies have evolved significantly. For instance, microdosed ACTH patches with transdermal absorption capabilities are being developed to reduce systemic corticosteroid side effects. Oral dispersible tablets of vigabatrin are now available for infants who cannot tolerate injections or capsules, offering improved administration and compliance rates. Additionally, several companies are testing intranasal formulations for faster systemic absorption.
Wearable monitoring devices, particularly those incorporating AI and motion analysis, are being integrated into pediatric care workflows. These devices enable remote detection of spasms, reduce emergency hospital visits, and support long-term treatment planning. Cloud-based dashboards aggregate seizure data and provide actionable insights to neurologists in real time.
Digital therapeutics platforms now include caregiver coaching modules, teleconsultation portals, and remote dose adjustment systems. These technologies not only improve patient adherence but also enhance clinical oversight. The convergence of pharmacological innovation and smart technology continues to transform the Infantile Spasm Treatment Market into a more responsive and data-driven ecosystem.
• In March 2024, Aquestive Therapeutics initiated Phase 3 trials for a new oral film formulation of vigabatrin designed for pediatric patients, aiming to simplify dosing and reduce gastrointestinal side effects often associated with traditional tablets.
• In January 2024, a pediatric neurology network in the U.S. deployed an AI-powered EEG interpretation system that reduced manual review time by 42% and improved early spasm detection accuracy by 28% in children under one year of age.
• In July 2023, Mallinckrodt Pharmaceuticals completed a clinical-scale rollout of a synthetic ACTH analog manufactured using solvent-free processes, lowering production waste by 31% compared to traditional formulations.
• In October 2023, Marinus Pharmaceuticals partnered with a wearable technology startup to co-develop a seizure monitoring patch capable of delivering real-time alerts and telemetry data to physicians through encrypted mobile apps.
The Infantile Spasm Treatment Market Report comprehensively analyzes the current and future dynamics of this specialized therapeutic domain. It covers an extensive range of market segments, including treatment types such as Adrenocorticotropic Hormone (ACTH), corticosteroids, vigabatrin, cannabidiol-based therapies, and emerging drug delivery platforms like oral dispersible films and transdermal systems. Applications span pediatric epilepsy treatment, rare neurogenetic disorders, tuberous sclerosis complex, and brain injury-associated spasms.
Geographically, the report offers insights into major markets including North America, Europe, Asia-Pacific, South America, and the Middle East & Africa, with detailed country-level analysis of growth drivers, regulatory environments, and infrastructure readiness. The end-user landscape includes hospitals, specialty clinics, rehabilitation centers, and homecare services, assessing their respective roles in diagnosis, therapy administration, and long-term care management.
The report also highlights critical technology developments, such as AI-enabled diagnostics, wearable monitoring devices, and smart medication adherence tools. It evaluates their impact on clinical workflows, patient outcomes, and market penetration strategies. Furthermore, it identifies strategic trends such as telehealth expansion, public-private healthcare investments, and shifts in payer frameworks.
By combining data-driven analysis with industry intelligence, this report equips stakeholders—including pharmaceutical firms, technology developers, healthcare providers, and policymakers—with the tools needed to make informed strategic decisions in a complex, high-impact pediatric market.
| Report Attribute / Metric | Report Details |
|---|---|
| Market Name | Global Infantile Spasm Treatment Market |
| Market Revenue (2024) | USD 380 Million |
| Market Revenue (2032) | USD 501.2 Million |
| CAGR (2025–2032) | 3.5 % |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User
|
| Key Report Deliverables | Revenue Forecast, Growth Trends, Technological Insights, Market Dynamics, Segment Analysis, Regional & Country-Wise Insights, Competitive Landscape, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Mallinckrodt Pharmaceuticals, Lundbeck, Upsher-Smith Laboratories, Sun Pharmaceutical Industries Ltd., Taro Pharmaceutical Industries Ltd., Aquestive Therapeutics, Zogenix, Inc., Marinus Pharmaceuticals, Inc., GW Pharmaceuticals, H. Lundbeck A/S, Teva Pharmaceutical Industries Ltd., Adamas Pharmaceuticals, Inc. |
| Customization & Pricing | Available on Request (10 % Customization is Free) |
