Reports
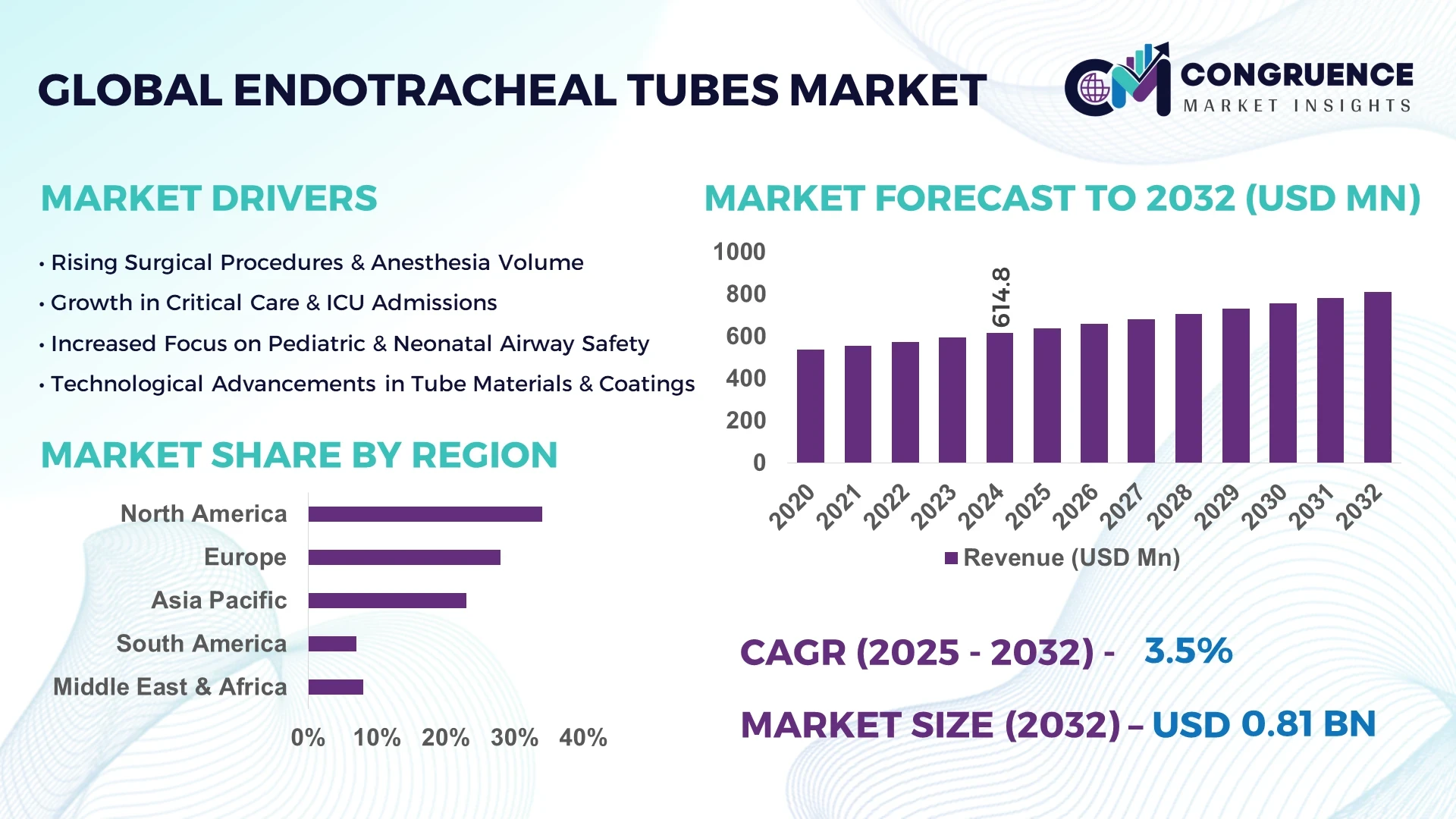
The Global Endotracheal Tubes Market was valued at USD 614.79 Million in 2024 and is anticipated to reach a value of USD 809.56 Million by 2032 expanding at a CAGR of 3.5% between 2025 and 2032.
India leads the global endotracheal tubes industry with a manufacturing infrastructure that includes state-of-the-art extrusion facilities and high-precision polymer processing units. The country’s manufacturing hubs have scaled up investment in ISO-certified production lines and advanced automation systems aimed at enhancing batch throughput and operational reliability. Industry applications span from tertiary hospitals and emergency transport units to rural healthcare centers, supported by rapid deployment of ergonomically designed, safety-focused tube systems, and continuous product upgrades complying with international sterilization standards.
The Endotracheal Tubes Market encompasses critical sectors such as hospital acute care, ambulatory surgery centers, emergency medical services, and specialized cancer and pediatric units. Recent innovations include antimicrobial-coated tubes that significantly reduce infection risk, sub-glottic secretion drainage designs, and bi-material cuff systems improving patient comfort and leak prevention. Regulatory mandates emphasizing single-use safety protocols alongside environmental considerations around biodegradable materials are driving material research and sustainable production methods. Regionally, adoption rates are rising fastest in Asia Pacific due to expanding surgical capacities and growing public health initiatives, whereas developed markets maintain steady demand through replacement cycles and technology upgrades. Emerging trends point to integration of smart monitoring features such as pressure sensors and digital connectors, alongside future outlooks focused on customization for difficult airway management and minimally invasive procedure compatibility—offering enhanced performance and clinical utility for healthcare decision-makers and industry professionals.
Artificial intelligence is reshaping the Endotracheal Tubes Market by introducing advanced quality control systems, predictive maintenance for manufacturing lines, and data-driven operational improvements. In production facilities, AI-driven visual inspection tools identify microscopic defects and surface irregularities in endotracheal tubes, reducing rejection rates and enhancing overall manufacturing efficiency. Smart sensors embedded in extrusion and assembly lines feed real-time data into machine-learning models that anticipate equipment wear, allowing preventative servicing and minimizing downtime. This optimization improves throughput consistency and operational uptime, which is vital for high-volume producers serving global healthcare networks.
Within logistical channels, AI-enhanced demand forecasting systems predict hospital procurement cycles and emergency response requirements, enabling manufacturers to align production with fluctuating needs—reducing inventory holding costs and ensuring timely delivery. AI-enabled maintenance scheduling has shown measurable gains, such as a reported 15% reduction in unplanned stoppages and a 12% increase in production uptime at select facilities implementing these tools. Additionally, smart packaging systems using AI image recognition ensure correct labeling, sterile packaging integrity, and compliance with regulatory traceability norms—boosting both safety and operational performance. These integrated AI applications strengthen the resilience and responsiveness of the Endotracheal Tubes Market, delivering quantifiable improvements in efficiency, product reliability, and process optimization tailored to supply-chain drivers and clinical stakeholders.
“In 2025, an Indian manufacturing facility deployed an AI-powered vision system that detected micro-level cuff defects in endotracheal tubes with 98.7% accuracy, reducing defect-related rejections by 20% within the first quarter of implementation.”
The Endotracheal Tubes Market is characterized by evolving clinical needs, technological innovation, and growing emphasis on patient safety and infection prevention. Increasing surgical volumes, driven by a rise in chronic respiratory diseases and emergency trauma cases, have heightened demand for advanced airway management solutions. Regulatory bodies are imposing stricter sterilization and material compliance requirements, influencing product design and manufacturing practices. Technological advancements, including antimicrobial coatings, subglottic suction features, and AI-integrated quality control, are shaping competitive differentiation. Additionally, regional healthcare infrastructure expansion in emerging economies is boosting procurement, while mature markets focus on premium, customized products. The interplay of innovation, compliance, and healthcare demand continues to define the growth trajectory of the Endotracheal Tubes Market.
The rise in surgical interventions, particularly in orthopedics, cardiovascular treatments, and emergency trauma care, is significantly boosting demand in the Endotracheal Tubes Market. According to industry data, global surgical procedures exceed 300 million annually, with a consistent upward trend due to aging populations and increased access to advanced healthcare in emerging regions. Endotracheal tubes are indispensable in ensuring airway management during anesthesia, making their usage proportional to surgical frequency. The introduction of specialized tubes for pediatric, geriatric, and complex airway cases has broadened application scope. As more hospitals adopt advanced operating room equipment, the demand for high-performance, safety-compliant endotracheal tubes is expected to sustain momentum, supported by continuous improvements in cuff design and material biocompatibility.
The Endotracheal Tubes Market faces constraints due to stringent regulatory frameworks governing medical device approval and manufacturing standards. Compliance with ISO, CE, and FDA guidelines requires extensive testing, clinical validation, and documentation, which can extend product launch timelines by months or even years. These requirements, while ensuring safety and performance, increase R&D and certification costs, creating barriers for smaller manufacturers. Moreover, periodic regulatory updates necessitate ongoing adjustments to manufacturing processes, adding complexity to operations. This can slow innovation cycles, delay market entry for new designs, and limit the ability of companies to quickly adapt to evolving clinical demands, impacting overall market responsiveness.
The adoption of smart sensor-enabled endotracheal tubes presents a significant growth opportunity in the Endotracheal Tubes Market. Embedded sensors can monitor cuff pressure, detect secretion levels, and provide real-time alerts to clinicians, reducing complications such as ventilator-associated pneumonia. Hospitals increasingly prioritize connected medical devices for integration into electronic health records and centralized patient monitoring systems, enhancing workflow efficiency. Industry pilots have demonstrated that smart monitoring features can reduce airway-related adverse events by up to 25%. As the Internet of Medical Things (IoMT) ecosystem expands, manufacturers leveraging sensor integration and wireless communication capabilities can position themselves at the forefront of high-value, technology-driven airway management solutions.
One of the major challenges in the Endotracheal Tubes Market is the escalating cost of raw materials, particularly medical-grade polymers such as polyvinyl chloride (PVC) and silicone. Volatility in global supply chains, driven by geopolitical tensions and energy price fluctuations, has increased procurement expenses for manufacturers. Additionally, specialized antimicrobial coatings and advanced cuff materials further raise production costs. Coupled with growing labor costs and investments required for automation and quality control systems, these factors can compress profit margins. Smaller producers, in particular, struggle to maintain competitive pricing while meeting stringent performance and safety standards, posing a significant barrier to scalability and market penetration.
Adoption of Antimicrobial-Coated Endotracheal Tubes: The incorporation of antimicrobial coatings, such as silver or specialized polymer-based agents, is gaining traction to reduce ventilator-associated infections. Clinical evaluations have shown up to a 40% reduction in bacterial colonization when using these coated tubes compared to standard variants. Hospitals and critical care units are prioritizing these solutions as part of broader infection control strategies. Manufacturers are increasingly integrating such coatings into both standard and specialized tube designs, enhancing market competitiveness while aligning with global healthcare safety mandates.
Integration of Subglottic Secretion Drainage Systems: Endotracheal tubes with integrated subglottic secretion drainage (SSD) systems are seeing accelerated adoption due to their proven ability to reduce the incidence of ventilator-associated pneumonia. Studies indicate SSD-equipped tubes can lower infection rates by up to 50% in long-term ventilation cases. This trend is particularly pronounced in intensive care units and emergency transport services, where patient outcomes and reduced hospital stays are key performance metrics.
Development of Specialized Pediatric Endotracheal Tubes: Manufacturers are focusing on ergonomically designed, size-specific pediatric tubes to address airway management challenges in neonatal and pediatric surgeries. Enhanced cuff technology and flexible materials are improving fit and reducing mucosal injury risks. The growing number of pediatric surgical procedures and advancements in minimally invasive pediatric care are contributing to increased demand for such specialized devices.
Emergence of Smart Monitoring Capabilities: Smart endotracheal tubes embedded with sensors capable of measuring cuff pressure and airflow are entering commercial production. Early deployment in pilot hospitals has demonstrated real-time monitoring benefits, including a 25% reduction in airway-related complications. The integration with central monitoring stations enables clinicians to respond promptly to pressure fluctuations, enhancing patient safety and optimizing ventilation management.
The Endotracheal Tubes Market is segmented by type, application, and end-user, reflecting a diverse demand structure across clinical environments. Type-based segmentation includes standard tubes, reinforced tubes, and specialized variants such as armored or laser-resistant models, each serving specific airway management needs. Application segmentation spans surgical procedures, emergency care, and long-term ventilation, with adoption patterns influenced by clinical protocols and patient demographics. End-user segmentation covers hospitals, ambulatory surgical centers, and emergency medical services, where purchasing decisions are driven by equipment compatibility, safety standards, and procedural frequency. Understanding these segments is essential for manufacturers and distributors to tailor product offerings, enhance innovation, and meet the evolving needs of healthcare systems worldwide.
Standard endotracheal tubes represent the leading type due to their wide applicability in both elective and emergency surgical procedures, offering a cost-effective and readily available solution for airway management. These tubes are used extensively in hospitals globally, making them a consistent driver of market volume. The fastest-growing segment is reinforced endotracheal tubes, designed with embedded spiral wiring to prevent kinking during complex surgeries or patient positioning changes. Their adoption is expanding in neurosurgical, ENT, and maxillofacial operations where airway stability is critical. Specialized types, such as laser-resistant tubes used in airway surgeries involving laser procedures, and double-lumen tubes for thoracic operations, cater to niche clinical scenarios. Additionally, cuffed pediatric and uncuffed variants are gaining ground in pediatric care, addressing airway safety concerns in younger patients. This diversified product portfolio ensures that the Endotracheal Tubes Market can meet a wide spectrum of procedural requirements.
Surgical procedures dominate the Endotracheal Tubes Market application segment due to the consistent requirement for airway control during anesthesia. These tubes are indispensable in cardiovascular, orthopedic, and general surgeries, ensuring uninterrupted ventilation and patient safety. Emergency care is the fastest-growing application, fueled by the rise in trauma cases, critical care interventions, and the expansion of advanced emergency medical services. The increasing deployment of pre-hospital airway management protocols by trained paramedics and first responders has elevated demand for portable, high-durability tubes. Long-term ventilation applications, often used in intensive care units for patients requiring extended respiratory support, remain a vital segment, with ongoing innovation in cuff materials and secretion management systems aimed at reducing complications. Each application area continues to evolve alongside advancements in critical care protocols, directly influencing procurement patterns.
Hospitals are the leading end-users in the Endotracheal Tubes Market due to their comprehensive surgical capacity, intensive care facilities, and higher procedural volumes. These institutions require diverse tube types to accommodate a wide range of surgeries and critical care scenarios, driving bulk procurement. Emergency medical services are the fastest-growing end-user segment, propelled by enhanced ambulance equipment standards, increased training for field airway management, and the adoption of advanced airway devices in pre-hospital settings. Ambulatory surgical centers also contribute significantly, especially in developed markets where outpatient procedures are increasing. These centers prioritize lightweight, easy-to-handle tubes with high safety ratings. Other end-users, including military medical units and specialized clinics, maintain consistent niche demand, particularly for mission-critical or high-risk environments. Together, these segments reflect the varied operational needs shaping product development and distribution strategies in the Endotracheal Tubes Market.
North America accounted for the largest market share at 34% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 5.1% between 2025 and 2032.
The market dynamics in these regions are shaped by healthcare infrastructure maturity, surgical demand, and investment in innovative airway management solutions. North America benefits from advanced healthcare systems and high surgical volumes, while Asia-Pacific’s growth is driven by expanding hospital networks, rising medical tourism, and manufacturing capacity. Europe remains a stable and technologically progressive market, South America shows moderate growth potential with emerging procurement reforms, and the Middle East & Africa are increasingly investing in advanced medical equipment to support expanding healthcare projects.
Advanced Airway Management Driving Clinical Efficiency
Holding 34% of the global market in 2024, this region’s demand is powered by a robust hospital network, increasing surgical volumes, and significant adoption of advanced airway devices in intensive care. The presence of leading medical device manufacturers ensures rapid access to product innovations such as antimicrobial-coated and sensor-integrated tubes. Regulatory frameworks encourage continuous improvement in patient safety standards, with updated medical device quality compliance requirements. Digital transformation is accelerating with AI-based manufacturing quality checks and connected airway monitoring systems, ensuring efficiency in both production and clinical use.
Innovation and Safety Compliance Leading Adoption
Accounting for approximately 28% of the global volume in 2024, this region is supported by strong healthcare systems in Germany, the UK, and France. Regulatory bodies such as the European Medicines Agency and national health authorities have implemented stricter quality compliance standards, driving adoption of high-performance airway devices. Sustainability initiatives are influencing material selection, with increased interest in eco-friendly, single-use designs. Technological uptake is strong, with hospitals adopting laser-resistant tubes, subglottic drainage systems, and antimicrobial coatings to improve clinical outcomes and meet evolving patient safety benchmarks.
Expanding Healthcare Access Driving Device Adoption
Representing 23% of the market volume in 2024, this region is the fastest-growing due to rising surgical capacities in China, India, and Japan. Rapid infrastructure development is boosting hospital bed counts and intensive care capacity, while domestic manufacturing hubs in China and India are scaling production of cost-effective and advanced endotracheal tubes. Innovation clusters in Japan and South Korea are leading research into smart airway devices and patient-specific designs. Increasing medical tourism in countries like Thailand and Malaysia is also fueling procurement of advanced airway management equipment.
Healthcare Modernization Boosting Procurement Rates
Capturing nearly 7% of global market share in 2024, this region is led by Brazil and Argentina, where investments in hospital infrastructure and emergency response systems are rising. Modernization of healthcare delivery is boosting demand for safer, more efficient airway management devices. Government-backed programs are supporting medical equipment imports and promoting domestic manufacturing partnerships. Growing adoption of specialized endotracheal tubes in trauma care and emergency transport is enhancing clinical readiness across major urban centers.
Strategic Healthcare Investments Fueling Demand
Holding about 8% of the global market in 2024, this region is experiencing increasing demand from the UAE, Saudi Arabia, and South Africa. Expanding hospital capacity, coupled with large-scale government healthcare projects, is driving procurement of advanced airway devices. Local regulations emphasize alignment with global safety standards, prompting adoption of antimicrobial and laser-resistant tube designs. Technological modernization is evident through integration of AI-powered monitoring systems and remote clinical training programs to enhance device handling skills among medical staff.
United States – 28% market share – Strong clinical demand driven by high surgical volumes, advanced intensive care infrastructure, and rapid adoption of smart airway devices.
China – 15% market share – High production capacity combined with expanding domestic hospital networks and growing export of cost-effective, advanced endotracheal tubes.
The Endotracheal Tubes Market is characterized by a moderately consolidated competitive landscape with over 40 active global and regional manufacturers supplying to hospitals, surgical centers, and emergency medical services. Leading players maintain strong market positioning through diversified product portfolios, advanced manufacturing capabilities, and extensive global distribution networks. Strategic initiatives such as joint ventures, regional manufacturing partnerships, and targeted acquisitions have been instrumental in expanding market reach and accelerating innovation. In the past two years, there has been a notable increase in product launches featuring antimicrobial coatings, integrated subglottic suction systems, and smart monitoring features to address evolving clinical needs. Competitive differentiation is increasingly driven by compliance with stringent regulatory standards and the ability to offer customized solutions for specific surgical and emergency scenarios. Companies are also investing in automation, AI-driven quality control, and sustainable materials to enhance efficiency, reduce costs, and align with environmental goals. This evolving competitive environment continues to push manufacturers toward advanced technology adoption and value-added product offerings.
Medtronic plc
Teleflex Incorporated
Smiths Medical (ICU Medical, Inc.)
Medline Industries, LP
Bactiguard AB
Flexicare Medical Ltd.
Armstrong Medical Ltd.
Vyaire Medical, Inc.
Parker Medical, Inc.
Intersurgical Ltd.
The Endotracheal Tubes Market is undergoing significant technological evolution, with advancements aimed at improving patient safety, reducing infection risks, and enhancing clinical efficiency. One major innovation trend is the adoption of antimicrobial-coated tubes, which use silver ions or polymer-based agents to inhibit bacterial colonization and lower incidences of ventilator-associated pneumonia. Laboratory studies indicate that these coatings can reduce microbial growth by up to 99% within the first 24 hours of intubation.
Another impactful technology is the integration of subglottic secretion drainage systems (SSD), which allow continuous removal of secretions above the cuff, significantly lowering infection risks in long-term ventilation cases. This feature has been shown to reduce airway-related complications by nearly 50% in intensive care settings. The development of smart monitoring-enabled endotracheal tubes is also advancing rapidly. These devices feature embedded micro-sensors that track cuff pressure, airflow resistance, and patient breathing patterns in real time, transmitting data to central monitoring stations for immediate clinical response.
Material innovations are equally important, with manufacturers exploring biocompatible, biodegradable, and latex-free polymers to address both patient safety and environmental sustainability concerns. Additionally, automated manufacturing lines incorporating AI-driven inspection systems are improving production accuracy, reducing defect rates, and ensuring compliance with stringent quality regulations. Collectively, these technologies are shaping a next-generation product landscape that aligns with the demands of high-performance healthcare environments.
In March 2024, Medtronic launched a new antimicrobial-coated endotracheal tube series designed to reduce bacterial colonization, with early clinical trials showing a 38% decrease in ventilator-associated pneumonia rates in ICU patients.
In September 2024, Teleflex introduced a reinforced endotracheal tube with enhanced kink resistance for complex surgical positioning, targeting neurosurgery and maxillofacial procedures.
In May 2023, Bactiguard AB expanded its production capacity in Asia with a new facility dedicated to antimicrobial medical devices, including endotracheal tubes, enabling faster regional distribution.
In November 2023, Vyaire Medical unveiled a smart monitoring-enabled endotracheal tube prototype capable of transmitting real-time cuff pressure data, aiming to improve ventilation safety in critical care units.
The Endotracheal Tubes Market Report provides a comprehensive analysis of the industry, covering product types, applications, end-users, and geographic regions. The type segmentation examines standard, reinforced, laser-resistant, and specialized pediatric endotracheal tubes, detailing their clinical uses and adoption rates. Application coverage includes surgical procedures, emergency care, and long-term ventilation, each assessed for procedural demand patterns and innovation-driven shifts. Geographically, the report analyzes five key regions—North America, Europe, Asia-Pacific, South America, and the Middle East & Africa—highlighting country-level trends, consumption volumes, and manufacturing capabilities. Particular attention is given to high-demand countries such as the United States, China, India, Germany, and Brazil, which influence global supply and adoption patterns.
Technological focus areas include antimicrobial coatings, subglottic secretion drainage systems, smart monitoring capabilities, and sustainable material development. The report also examines how regulatory compliance, healthcare infrastructure investments, and digital transformation trends are shaping market competition and innovation pipelines. Additionally, the scope extends to niche growth areas such as specialized tubes for laser surgery, double-lumen designs for thoracic procedures, and biocompatible eco-friendly variants. By offering insights across diverse market drivers, challenges, and emerging opportunities, the report equips decision-makers with actionable intelligence for strategic planning and market positioning in the evolving global Endotracheal Tubes industry.
| Report Attribute/Metric | Report Details |
|---|---|
|
Market Revenue in 2024 |
USD 614.79 Million |
|
Market Revenue in 2032 |
USD 809.56 Million |
|
CAGR (2025 - 2032) |
3.5% |
|
Base Year |
2024 |
|
Forecast Period |
2025 - 2032 |
|
Historic Period |
2020 - 2024 |
|
Segments Covered |
By Types
By Application
By End-User
|
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Region Covered |
North America, Europe, Asia-Pacific, South America, Middle East, Africa |
|
Key Players Analyzed |
Medtronic plc, Teleflex Incorporated, Smiths Medical (ICU Medical, Inc.), Medline Industries, LP, Bactiguard AB, Flexicare Medical Ltd., Armstrong Medical Ltd., Vyaire Medical, Inc., Parker Medical, Inc., Intersurgical Ltd. |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
