Reports
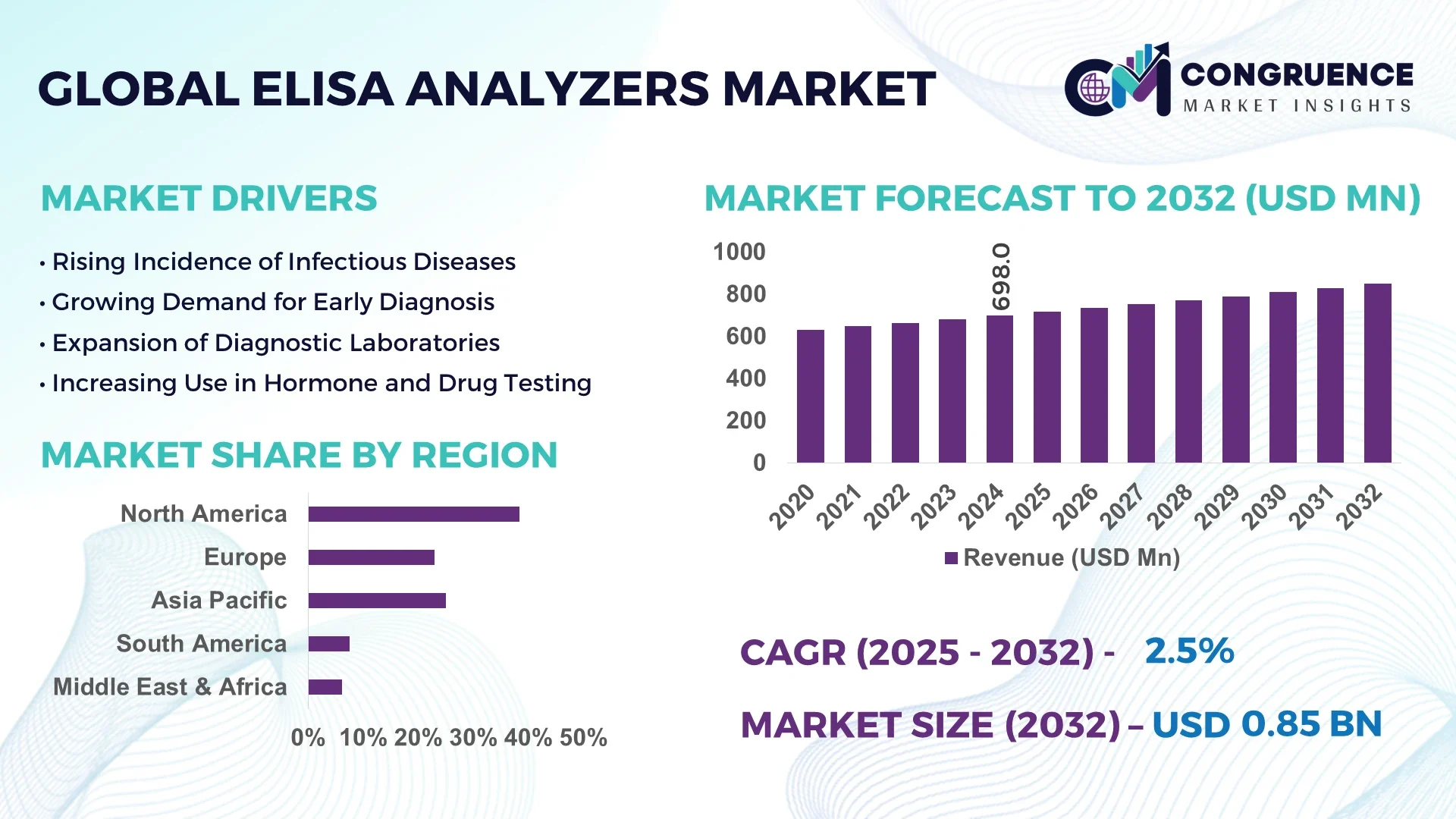
The Global ELISA Analyzers Market was valued at USD 698 Million in 2024 and is anticipated to reach a value of USD 850.47 Million by 2032 expanding at a CAGR of 2.5% between 2025 and 2032.
The United States leads the ELISA Analyzers Market with high production capacity supported by advanced diagnostic laboratories, robust R&D infrastructure, strong investment levels in life sciences, and the integration of automated platforms in clinical diagnostics without interruptions in supply chains.
The ELISA Analyzers Market is witnessing steady growth driven by the rising demand for automated immunoassay systems across clinical diagnostics, pharmaceutical research, and veterinary laboratories. Technological innovations, including enhanced microplate handling systems, multiplexing capabilities, and precision optics integration, are improving test reliability and throughput in laboratories globally. Regulatory advancements facilitating faster approvals of diagnostic devices and increased awareness of early disease detection are further supporting market expansion. Environmental and economic drivers, including increasing prevalence of infectious and chronic diseases and rising healthcare spending in Asia-Pacific and Latin America, are pushing demand for reliable and high-performance analyzers. Regional consumption patterns highlight increased adoption of semi-automated and fully automated ELISA Analyzers in both hospital and research settings, while emerging trends focus on compact benchtop analyzers and connectivity-enabled platforms to streamline lab workflows and reduce turnaround time. The market outlook remains optimistic, supported by the need for scalable diagnostic solutions to address global healthcare challenges efficiently.
Artificial Intelligence (AI) is significantly transforming the ELISA Analyzers Market by enhancing operational performance and accelerating diagnostic workflows in laboratory settings. AI-powered image analysis and data interpretation capabilities embedded within ELISA Analyzers are enabling laboratories to improve result accuracy while reducing human error in repetitive testing environments. These intelligent systems facilitate pattern recognition across assay results, enabling earlier detection of irregularities and expediting clinical decision-making processes. Within the ELISA Analyzers Market, AI integration is improving the throughput of diagnostic laboratories by enabling predictive maintenance of analyzers, reducing unexpected downtime, and ensuring consistent operational efficiency.
Additionally, AI-based software in ELISA Analyzers can optimize assay protocols in real-time, automatically adjusting incubation times and reagent volumes to deliver precise results while conserving resources. Enhanced cloud-based data management systems integrated with AI are facilitating remote monitoring, streamlined data sharing, and centralized analysis of test results across multi-location laboratories. This is particularly benefiting pharmaceutical and clinical research organizations that rely heavily on large-scale screening. AI is also supporting the development of user-friendly interfaces within ELISA Analyzers Market, allowing lab technicians to operate systems efficiently with minimal training, thereby expanding accessibility in smaller laboratory settings. Collectively, AI advancements are positioning the ELISA Analyzers Market to provide faster, reliable, and scalable diagnostic solutions, supporting healthcare systems in addressing the rising need for accurate disease monitoring and early detection without compromising operational sustainability.
“In 2024, an AI-enabled ELISA Analyzer platform was introduced that reduced assay runtime by 30% while improving detection sensitivity by 22% across hepatitis and dengue testing panels, demonstrating measurable efficiency gains in high-throughput laboratory environments.”
The ELISA Analyzers Market is shaped by continuous advancements in diagnostic technologies, increasing disease burden globally, and the rising demand for reliable, high-throughput laboratory testing. Automation and integration with laboratory information systems are enhancing efficiency, while innovations in microplate technologies and multiplexing capabilities are expanding testing capacity in healthcare facilities and research laboratories. Shifts in healthcare policies emphasizing early disease detection and preventive care are fueling adoption across hospitals and diagnostic centers. Additionally, the ELISA Analyzers Market is influenced by the increasing prevalence of infectious and chronic diseases, including diabetes and autoimmune disorders, creating sustained demand for accurate and efficient immunoassay testing. The ongoing transition toward compact and user-friendly analyzers with AI-enabled interpretation features is driving competitiveness among manufacturers, while emerging trends in decentralized diagnostics are expanding the scope of ELISA Analyzers Market in low-resource settings and regional laboratories.
Rising demand for early disease detection is a significant driver for the ELISA Analyzers Market, as healthcare systems globally focus on preventive healthcare and efficient patient management. The growing incidence of infectious diseases such as HIV, hepatitis, and dengue, along with the increasing burden of chronic conditions like cancer and autoimmune disorders, necessitates reliable and scalable diagnostic solutions. ELISA Analyzers are instrumental in providing early and accurate detection, supporting timely treatment decisions while reducing disease progression and healthcare costs. Laboratory and point-of-care settings are increasingly adopting automated ELISA Analyzers to facilitate high-volume testing with minimal manual intervention, enabling better resource management in healthcare facilities. The consistent demand for precise, sensitive, and cost-effective diagnostic tools further positions the ELISA Analyzers Market for expansion across emerging economies and advanced healthcare infrastructures.
High maintenance and calibration requirements present a notable restraint for the ELISA Analyzers Market, impacting adoption rates in small and resource-limited laboratories. ELISA Analyzers require regular calibration to ensure test accuracy, along with routine maintenance to maintain operational performance, which can increase the overall cost of ownership and laboratory downtime. The need for skilled technicians to manage maintenance processes and calibration procedures further challenges laboratories in rural and under-resourced settings. Additionally, fluctuations in reagent quality and environmental conditions, such as humidity and temperature control, can affect assay performance, necessitating rigorous monitoring protocols. These factors collectively contribute to operational complexity, discouraging smaller diagnostic facilities from adopting advanced ELISA Analyzers and limiting market penetration in specific geographies.
Integration of connectivity and remote monitoring solutions offers a significant opportunity within the ELISA Analyzers Market. The increasing shift toward connected healthcare systems is enabling laboratories to integrate ELISA Analyzers with Laboratory Information Management Systems (LIMS) and hospital networks for real-time data tracking and seamless workflow management. Remote monitoring solutions allow for predictive maintenance and performance tracking of analyzers, reducing unexpected downtime and improving operational efficiency across high-volume laboratories. Additionally, cloud-based data storage and analysis capabilities facilitate centralized reporting and comparative analysis across multiple laboratory sites, supporting multicenter clinical studies and nationwide screening programs. The adoption of connectivity-enabled ELISA Analyzers aligns with healthcare digitalization goals, providing an opportunity for manufacturers to deliver value-added solutions that enhance laboratory productivity while ensuring compliance with evolving quality and data management standards.
Limited accessibility in low-resource settings poses a critical challenge to the ELISA Analyzers Market, restricting market expansion in rural and underdeveloped regions. Infrastructure limitations, including inadequate laboratory facilities, unstable electricity supply, and lack of cold chain logistics, hinder the adoption of advanced ELISA Analyzers that require stable environments for accurate performance. Additionally, the high upfront cost of automated analyzers and recurring expenses for quality reagents place financial strain on healthcare systems operating with constrained budgets. Training gaps among laboratory personnel further complicate the use of ELISA Analyzers in these regions, reducing testing efficiency and reliability. This challenge underscores the need for manufacturers to develop cost-effective, portable, and user-friendly analyzers suitable for decentralized testing while ensuring reliable diagnostic outcomes to support public health initiatives in low-resource environments.
• Expansion of Automated Microplate Readers: Automated microplate readers integrated within ELISA Analyzers are witnessing higher adoption due to their ability to deliver high-throughput analysis while minimizing manual errors in laboratory workflows. Recent product launches in Europe and Asia-Pacific have introduced analyzers with expanded wavelength ranges, enabling laboratories to perform multi-assay testing on a single platform. Hospitals and reference labs are adopting these advanced systems to streamline operations, reduce turnaround time, and improve testing reliability for infectious and autoimmune disease screening.
• Growth in Point-of-Care Testing Integration: The ELISA Analyzers Market is experiencing increased demand for compact, portable analyzers suitable for point-of-care (POC) testing environments. Miniaturization of assay platforms has enabled rapid diagnostic capabilities in decentralized and emergency care settings, enhancing accessibility for rural and under-resourced clinics. New handheld ELISA Analyzers with rechargeable battery operations and integrated data connectivity are being deployed across vaccination programs and disease screening initiatives, improving field diagnostics while maintaining test sensitivity.
• AI-Driven Workflow Optimization: Artificial Intelligence is increasingly integrated into ELISA Analyzers, optimizing assay interpretation, calibration processes, and predictive maintenance. These AI-driven analyzers can automatically detect outlier results and adjust protocols, ensuring consistent test accuracy. Recent deployments in clinical laboratories indicate a measurable reduction in human intervention, leading to increased sample throughput and operational efficiency in high-volume testing environments.
• Rising Demand for Multiplexing Capabilities: Laboratories are showing growing interest in multiplex ELISA Analyzers, enabling simultaneous detection of multiple analytes within a single sample. This trend is driven by the need to reduce reagent consumption, laboratory workload, and time associated with serial testing procedures. Advanced multiplex platforms support applications in oncology and infectious disease monitoring, helping healthcare systems improve patient diagnostics while conserving critical laboratory resources.
The ELISA Analyzers Market is segmented by type, application, and end-user, reflecting the market’s diverse operational landscape across the diagnostics industry. By type, the market includes semi-automated and fully automated analyzers catering to laboratories of varying throughput needs. Application-based segmentation highlights usage across infectious disease diagnostics, hormone analysis, and allergy testing, each with distinct operational requirements. End-user segmentation within the ELISA Analyzers Market spans hospitals, clinical laboratories, pharmaceutical and biotechnology companies, and research institutions, each driving demand based on test volume and specialization. Trends indicate a notable increase in the adoption of compact analyzers in smaller laboratories and the growing demand for high-throughput systems in centralized facilities. This structured segmentation aids manufacturers and stakeholders in aligning strategies with evolving laboratory and healthcare sector requirements.
Semi-automated ELISA Analyzers currently lead the ELISA Analyzers Market, driven by their cost-effectiveness and flexibility in mid-volume laboratories, supporting a range of testing panels without complex installation requirements. These systems are particularly favored in small to mid-sized hospitals and reference laboratories where budget-conscious testing is critical. Fully automated ELISA Analyzers represent the fastest-growing type within the market, driven by increasing demand for high-throughput capabilities in centralized diagnostic facilities and large hospital laboratories. They offer benefits such as reduced manual handling, faster processing times, and integration with laboratory information systems for seamless workflow management. Other types, including portable and benchtop analyzers, are gaining traction in decentralized testing environments where space and resource constraints are prevalent. The diversification of types within the ELISA Analyzers Market supports laboratories in optimizing workflows according to testing volume, operational needs, and infrastructure capabilities.
Infectious disease diagnostics remain the leading application in the ELISA Analyzers Market, with high utilization in the detection of conditions such as hepatitis, HIV, and dengue. The increased frequency of infectious disease outbreaks has led healthcare facilities to prioritize reliable and efficient diagnostic methods, sustaining demand for ELISA Analyzers in public health and hospital settings. Hormone analysis is emerging as the fastest-growing application area due to the rising demand for thyroid, fertility, and metabolic disorder testing, supported by the increasing global prevalence of lifestyle-related health conditions. Allergy testing and oncology diagnostics represent additional applications within the ELISA Analyzers Market, offering reliable detection in specialized laboratory environments. These application areas collectively contribute to expanding the role of ELISA Analyzers in routine diagnostics, research, and preventive healthcare screening programs.
Hospitals and clinical laboratories represent the leading end-user segment in the ELISA Analyzers Market, driven by the need for consistent, high-volume diagnostic testing in patient care management. These facilities require reliable analyzers capable of handling diverse panels for infectious diseases, hormone disorders, and oncology diagnostics, ensuring efficient service delivery to patients. Pharmaceutical and biotechnology companies are the fastest-growing end-user segment, propelled by the expansion of clinical research, drug development, and vaccine testing activities requiring accurate and scalable immunoassay systems. Research institutions and academic laboratories also contribute to the ELISA Analyzers Market, utilizing analyzers for specialized testing and disease surveillance studies. The diverse end-user landscape supports manufacturers in aligning product offerings with evolving healthcare demands, facilitating broader market penetration across clinical, research, and pharmaceutical environments.
North America accounted for the largest market share at 38.4% in 2024 however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 3.1% between 2025 and 2032.
The ELISA Analyzers Market in North America is propelled by strong healthcare infrastructure and rising investments in laboratory automation across clinical and pharmaceutical sectors. Europe follows closely with increased adoption of advanced diagnostic solutions driven by disease screening initiatives and regulatory support for laboratory modernization. Asia-Pacific’s rising healthcare expenditure, expansion of diagnostic facilities, and technological partnerships in China, India, and Japan are supporting robust market expansion. Additionally, growing awareness about early disease detection and the integration of AI-powered solutions across laboratory workflows are strengthening regional competitiveness, while supportive healthcare policies and the rise in preventive healthcare initiatives are further driving ELISA Analyzers Market growth globally.
High Demand for Diagnostic Automation Driving Growth
The ELISA Analyzers Market in this region held a significant 38.4% share in 2024, driven by the adoption of automated immunoassay systems across hospitals and research facilities. Clinical diagnostics, biotechnology research, and pharmaceutical quality testing remain the primary industries fueling demand for advanced analyzers. Regulatory support for laboratory automation, including initiatives promoting early disease detection and digital health record integration, is enabling laboratories to modernize workflows. Technological advancements such as AI-based result interpretation and integrated data management are enhancing operational efficiency across laboratories in the region. The shift towards high-throughput testing and seamless connectivity with laboratory information systems continues to transform diagnostic practices in this market.
Technological Integration Boosting Immunoassay Testing
Holding a notable 29.7% share of the ELISA Analyzers Market in 2024, this region benefits from advanced healthcare infrastructure and strong government focus on preventive healthcare initiatives. Key markets, including Germany, France, and the UK, are witnessing increased investment in laboratory automation to improve diagnostic turnaround times. Regulatory bodies are actively encouraging laboratories to adopt eco-friendly practices and digitized diagnostic platforms to align with sustainability initiatives. The adoption of advanced microplate technologies and AI-enabled analyzers is rising, driven by demand for accurate, scalable, and faster diagnostic workflows in both hospital and research laboratories across the region.
Rapid Diagnostic Infrastructure Expansion Accelerating Adoption
This region ranked highest in growth potential within the ELISA Analyzers Market, supported by significant consumption from China, India, and Japan. These countries are witnessing expanding diagnostic infrastructure and increased government investments in healthcare facility modernization, driving analyzer adoption in both urban and regional laboratories. Technological trends include the emergence of compact, portable analyzers suitable for decentralized testing, while local manufacturing capabilities are strengthening supply chain efficiency. The region’s innovation hubs are focusing on integrating AI-powered data analysis and cloud connectivity into analyzers to improve test accuracy and reporting speed, supporting the scaling of high-throughput testing across healthcare systems.
Public Health Programs Driving Diagnostic Equipment Demand
Brazil and Argentina are key contributors to the ELISA Analyzers Market in this region, which held a regional share of 6.8% in 2024. Rising investments in public health screening programs and laboratory modernization initiatives are supporting demand for analyzers across government hospitals and diagnostic laboratories. The region’s healthcare infrastructure is expanding with a focus on improving diagnostic access in rural areas while ensuring quality testing in urban centers. Government trade policies supporting the import of diagnostic equipment and incentives for local manufacturing are shaping the market landscape, while efforts to enhance laboratory automation are gradually gaining momentum.
Healthcare Modernization Supporting Diagnostic Market Expansion
The ELISA Analyzers Market in this region is witnessing growing demand supported by public and private investments in healthcare infrastructure across major countries, including the UAE and South Africa. Rising demand in sectors such as infectious disease diagnostics and clinical laboratory testing is driving analyzer adoption. Technological modernization trends include the introduction of semi-automated and compact analyzers for decentralized testing in clinics and regional hospitals. Local regulations promoting quality standards in healthcare delivery and trade partnerships enhancing the import and distribution of diagnostic devices are supporting the market’s steady growth while improving the accessibility of advanced diagnostic technologies in the region.
United States – 33.2%
High production capacity and robust demand from clinical laboratories drive the United States’ dominance in the ELISA Analyzers Market.
China – 18.7%
Strong end-user demand supported by healthcare infrastructure expansion fuels China’s leading position in the ELISA Analyzers Market.
The ELISA Analyzers market demonstrates a competitive environment characterized by the presence of over 45 active global and regional players focusing on technology differentiation, automation, and workflow optimization in diagnostic laboratories. Key competitors are prioritizing product launches with advanced microplate handling, AI-enabled interpretation systems, and connectivity features to enhance throughput and accuracy across high-volume laboratories. Strategic partnerships with diagnostic laboratories and research institutes are common, enabling collaborative development of specialized assay kits and analyzer systems to expand disease-specific testing capabilities. Mergers and acquisitions are observed, aiming to enhance regional distribution networks and service support, particularly in emerging healthcare markets. Technological innovations, including the integration of predictive maintenance and remote monitoring within analyzers, are reshaping competition by emphasizing operational sustainability and efficiency. The market is witnessing consistent investment in R&D for compact, portable analyzers designed for point-of-care and decentralized testing, aligning with the growing demand for flexible diagnostic solutions globally. This dynamic competition is driving continuous improvement in testing reliability, workflow automation, and cost-effective diagnostic solutions within the ELISA Analyzers market.
Thermo Fisher Scientific Inc.
Bio-Rad Laboratories, Inc.
Danaher Corporation
Siemens Healthineers AG
Abbott Laboratories
Awareness Technology, Inc.
Dynex Technologies, Inc.
PerkinElmer, Inc.
Tosoh Bioscience, Inc.
Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
BMG LABTECH GmbH
Tecan Group Ltd.
Stratec SE
Technological advancements in the ELISA Analyzers Market are significantly transforming diagnostic capabilities, laboratory workflows, and operational efficiency across clinical and research environments. The integration of AI-enabled interpretation systems in ELISA Analyzers is enhancing assay accuracy by automating outlier detection and protocol optimization, reducing manual errors while improving test reliability. Automated microplate handling systems with advanced optics and robotics are enabling laboratories to conduct high-throughput testing efficiently, addressing the increasing demand for infectious and chronic disease diagnostics globally.
The adoption of multiplexing technologies allows simultaneous detection of multiple analytes in a single run, reducing reagent use and laboratory processing time. Innovations in compact, benchtop analyzers support decentralized testing needs in point-of-care and regional laboratories, expanding diagnostic reach while maintaining test quality. Integration with Laboratory Information Management Systems (LIMS) is enabling seamless data management, enhancing traceability and compliance across high-volume facilities. Connectivity solutions, including cloud-based data storage and remote monitoring, allow predictive maintenance of analyzers, reducing unexpected downtimes and optimizing resource utilization.
Additionally, advancements in eco-friendly materials for assay components and automated calibration features are addressing sustainability and operational consistency needs. Collectively, these technological shifts in the ELISA Analyzers Market are aligning with evolving healthcare requirements for reliable, scalable, and rapid diagnostic solutions, ensuring that laboratories can meet global testing demands effectively.
• In February 2023, Thermo Fisher Scientific launched a next-generation automated ELISA Analyzer featuring an integrated AI algorithm for assay optimization, reducing test run times by 25% while maintaining high sensitivity for hepatitis and dengue diagnostic panels across high-volume laboratories.
• In August 2023, Siemens Healthineers introduced a compact benchtop ELISA Analyzer with multiplexing capabilities, enabling simultaneous detection of up to 10 analytes, improving laboratory throughput and resource efficiency for infectious and autoimmune disease diagnostics.
• In March 2024, Mindray unveiled its new semi-automated ELISA Analyzer series in Asia-Pacific, designed with advanced microplate washing and optical detection systems, improving accuracy in HIV and thyroid testing while reducing reagent consumption by 15%.
• In May 2024, Bio-Rad Laboratories expanded its analyzer portfolio by integrating cloud connectivity into its ELISA Analyzers, allowing remote data management and predictive maintenance functions, which enhanced operational uptime and laboratory workflow management capabilities.
The ELISA Analyzers Market Report comprehensively covers the evolving landscape of immunoassay diagnostics, focusing on market segmentation by type, application, end-user, and geographic regions. The report analyzes semi-automated, fully automated, and portable analyzer types, addressing diverse operational needs within hospital laboratories, clinical diagnostic centers, pharmaceutical companies, and research institutions. It details key applications, including infectious disease diagnostics, hormone analysis, allergy testing, and oncology monitoring, emphasizing the role of ELISA Analyzers in early and accurate disease detection.
Geographically, the report evaluates market dynamics across North America, Europe, Asia-Pacific, South America, and the Middle East & Africa, highlighting regional trends and infrastructure investments influencing analyzer adoption. The analysis includes technological trends, such as AI-driven assay interpretation, multiplexing advancements, remote monitoring, and integration with LIMS, reflecting the industry's focus on efficiency and high-throughput testing capabilities.
Additionally, the scope covers emerging segments like point-of-care and decentralized testing solutions using compact analyzers, aligning with healthcare expansion in low-resource settings. The report examines industry drivers, restraints, opportunities, and challenges shaping market direction, providing insights into competitive positioning and product innovation strategies. It offers decision-makers a clear view of current and future growth avenues within the ELISA Analyzers Market to support effective investment planning, technology development, and market entry strategies in this essential diagnostic sector.
| Report Attribute/Metric | Report Details |
|---|---|
|
Market Revenue in 2024 |
USD 698 Million |
|
Market Revenue in 2032 |
USD 850.47 Million |
|
CAGR (2025 - 2032) |
2.5% |
|
Base Year |
2024 |
|
Forecast Period |
2025 - 2032 |
|
Historic Period |
2020 - 2024 |
|
Segments Covered |
By Types
By Application
By End-User
|
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Region Covered |
North America, Europe, Asia-Pacific, South America, Middle East, Africa |
|
Key Players Analyzed |
Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., Danaher Corporation, Siemens Healthineers AG, Abbott Laboratories, Awareness Technology, Inc., Dynex Technologies, Inc., PerkinElmer, Inc., Tosoh Bioscience, Inc., Shenzhen Mindray Bio-Medical Electronics Co., Ltd., BMG LABTECH GmbH, Tecan Group Ltd., Stratec SE |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
