Reports
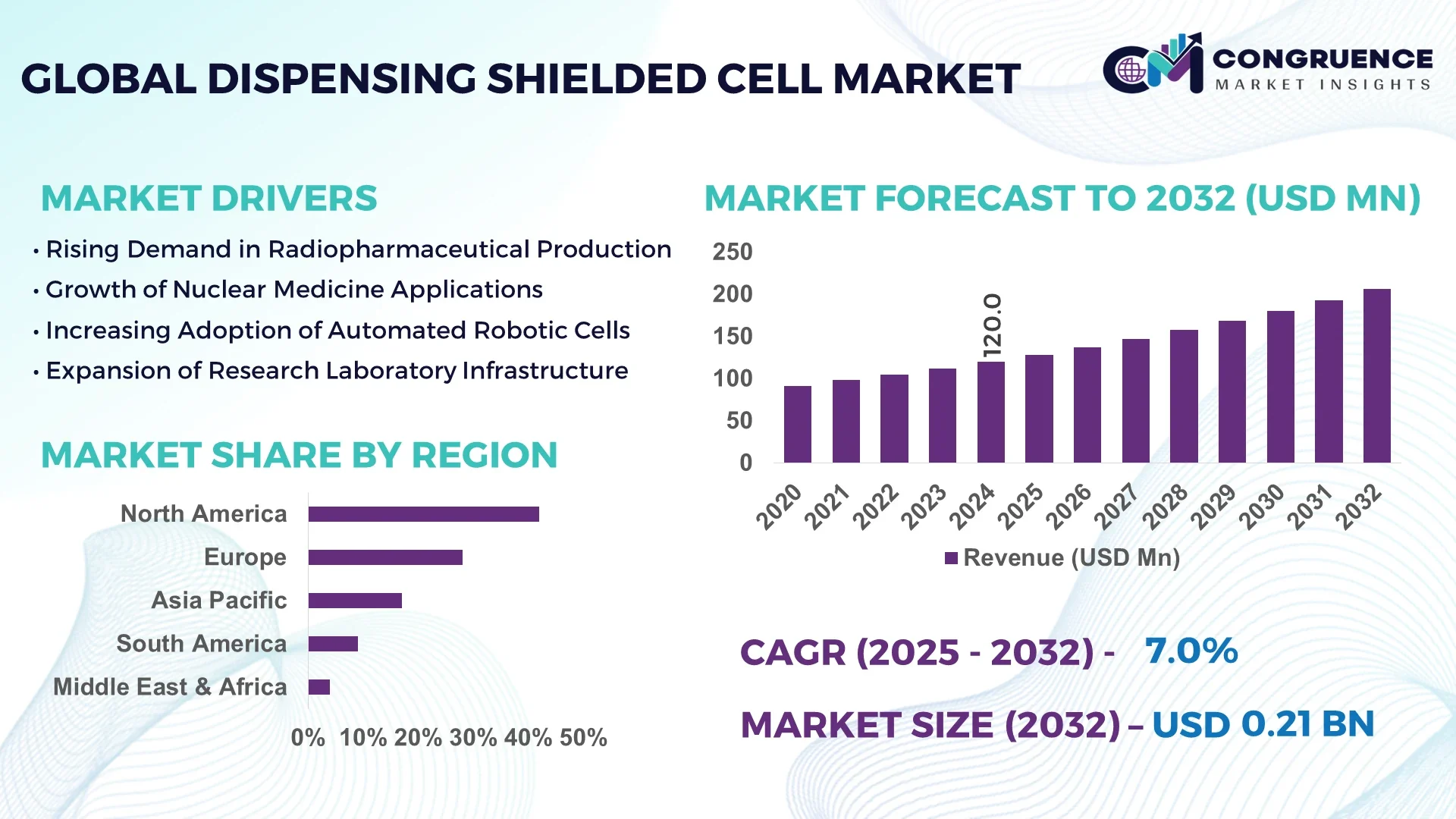
The Global Dispensing Shielded Cell Market was valued at USD 120 Million in 2024 and is anticipated to reach a value of USD 206.2 Million by 2032 expanding at a CAGR of 7.0% between 2025 and 2032.
The United States dominates the Dispensing Shielded Cell Market, with robust production capacity supported by significant investments in advanced containment systems. Leading manufacturers have established high-tech facilities for research, development, and large-scale production, supplying to pharmaceutical, biotech, and radiopharmaceutical industries. The country’s focus on automation, radiation safety standards, and integration of precision dispensing technologies further strengthens its market position.
The Dispensing Shielded Cell Market is witnessing rapid technological innovation, including automated radiation containment solutions, remote handling systems, and advanced shielding materials that improve operational safety and efficiency. Key industry sectors such as radiopharmaceutical manufacturing, nuclear medicine, and specialty chemical production are driving adoption. Regulatory requirements on radiation protection and workplace safety are influencing equipment design, while economic growth in healthcare and biotech is increasing demand for precise and reliable dispensing solutions. Regional consumption patterns show strong uptake in North America and Europe, with emerging growth in Asia-Pacific. Future trends include AI-driven monitoring, compact modular designs, and enhanced integration with laboratory automation platforms, shaping a more efficient, safe, and scalable market landscape.
AI is increasingly transforming the Dispensing Shielded Cell Market by enhancing operational performance, process optimization, and safety. Intelligent monitoring systems enable real-time detection of radiation exposure, fluid flow, and system integrity, allowing predictive maintenance and reducing downtime. AI-driven automation facilitates precise dispensing of radioactive or hazardous materials, minimizing human intervention and errors while improving consistency in production processes. Advanced image recognition and machine learning algorithms optimize shielding configurations, ensuring maximum safety and compliance with regulatory standards. In addition, AI enhances inventory management within shielded cell environments by predicting reagent usage, stock levels, and potential bottlenecks. By integrating AI with robotics, laboratories and production facilities can achieve fully automated, remote-controlled operations, significantly reducing exposure risk for operators. Moreover, data analytics generated by AI platforms provide actionable insights for decision-makers, enabling improved capacity planning, process improvements, and cost efficiency. The combination of AI and advanced containment technologies is revolutionizing the Dispensing Shielded Cell Market, enhancing both safety and productivity while supporting rapid innovation in pharmaceutical and radiopharmaceutical manufacturing.
“In 2024, a U.S.-based radiopharmaceutical facility implemented an AI-driven shielded cell monitoring system, reducing operator intervention time by 35% and detecting micro-leakages with 98% accuracy, enhancing overall process safety and efficiency.”
The Dispensing Shielded Cell Market is shaped by increasing demand for secure, automated handling of radioactive and hazardous materials. Key market trends include the adoption of modular and compact designs, integration with laboratory automation, and advanced shielding materials for enhanced safety. The market is influenced by regulatory standards, technological advancements, and growing requirements from radiopharmaceutical and biotech sectors. The rising need for precision dispensing, contamination prevention, and workflow optimization is driving continuous innovation, while regional growth patterns indicate high adoption in North America and Europe, with emerging markets in Asia-Pacific showing increasing investments in containment infrastructure.
The expansion of radiopharmaceutical production for diagnostics and therapeutics is a major growth driver. Increasing clinical and research activities in nuclear medicine and oncology are boosting the adoption of shielded dispensing systems. Manufacturers are responding with higher-capacity cells, automation integration, and improved shielding materials. Investments in safety protocols and remote handling solutions ensure compliance with strict radiation protection standards. Facilities handling sensitive radioactive isotopes require consistent, precise, and safe dispensing solutions, driving demand for advanced shielded cell technologies. Continuous innovation in containment design and material science further strengthens adoption, allowing operators to handle hazardous substances with minimal exposure risk.
The high capital expenditure required for advanced shielded cell systems remains a key restraint. Installation involves specialized infrastructure, lead shielding, and automation systems, which can be cost-prohibitive for smaller laboratories. Ongoing maintenance, regulatory compliance, and specialized operator training add to operational costs. Additionally, integration with robotic handling and AI monitoring systems requires technical expertise. Organizations in emerging regions may face challenges in financing and acquiring advanced containment technologies, slowing adoption rates despite growing industry demand. The combined effect of high upfront costs and complex operational requirements limits accessibility for some potential users.
Emerging markets present significant opportunities due to increasing investment in nuclear medicine, radiopharmaceutical manufacturing, and biotech research. Modular, prefabricated shielded cell designs allow rapid installation and scalability, catering to both new facilities and upgrades of existing infrastructure. Increasing government support for healthcare and research initiatives encourages adoption. Integration of automation and AI monitoring systems in these markets enables safer handling of radioactive materials and optimizes workflow efficiency. Additionally, environmentally friendly shielding materials and energy-efficient designs are gaining attention, providing opportunities for differentiation and adoption among forward-looking organizations.
The Dispensing Shielded Cell Market faces challenges related to stringent regulatory frameworks for radiation safety and containment. Compliance with multiple standards, certifications, and documentation requirements increases operational complexity. Rapid technological advancements require frequent system upgrades, staff retraining, and validation protocols, which can strain resources. Standardizing AI and automation integration across facilities remains challenging, particularly when retrofitting older infrastructure. Moreover, operators must balance safety, efficiency, and cost considerations while meeting local and international regulatory expectations. These challenges slow implementation and necessitate careful planning for market participants.
Rise in Modular and Prefabricated Construction: The adoption of modular construction is reshaping demand dynamics in the Dispensing Shielded Cell Market. Pre-bent and cut elements are prefabricated off-site using automated machines, reducing labor needs and speeding project timelines. Demand for high-precision machines is rising, especially in Europe and North America, where construction efficiency is critical.
AI-Driven Monitoring Systems: Advanced AI platforms are increasingly integrated into shielded cells to monitor radiation levels, fluid flow, and mechanical integrity in real time. These systems improve safety, optimize workflow efficiency, and reduce human intervention during high-risk operations.
Remote Handling and Robotics Adoption: Robotics for remote manipulation of hazardous or radioactive materials is becoming standard. This trend minimizes operator exposure and ensures consistent, precise dispensing while supporting automated laboratory processes.
Advanced Shielding Materials and Compact Designs: New high-density materials and compact configurations reduce space requirements while maintaining protection levels. Facilities are increasingly adopting lightweight, modular cells for improved workflow, energy efficiency, and operational flexibility.
The Dispensing Shielded Cell Market is segmented into types, applications, and end-users, each addressing specific operational requirements across pharmaceutical, radiopharmaceutical, and chemical industries. Segmentation enables stakeholders to identify precise equipment needs for containment, automation, and safety compliance. Product types vary in design, shielding efficiency, and automation capabilities, while applications range from routine dispensing to complex radiopharmaceutical synthesis. End-user insights highlight adoption patterns across hospitals, research laboratories, and industrial facilities. Understanding these segments helps decision-makers optimize investments, prioritize technological upgrades, and plan expansions effectively. Regional and operational considerations also play a crucial role in shaping product selection and deployment strategies.
The Dispensing Shielded Cell Market is primarily composed of Standard Shielded Cells, Automated/Robotic Cells, Modular Cells, and Customized Containment Systems. Standard Shielded Cells remain the leading type due to their versatility, reliability, and compatibility with a broad range of isotopes and hazardous materials. Automated or Robotic Shielded Cells represent the fastest-growing segment, driven by increasing demand for remote handling, operational efficiency, and precision in radiopharmaceutical dispensing. Modular Cells are gaining adoption for their flexibility in installation, scalability, and ease of maintenance, particularly in research and pilot-scale production environments. Customized Containment Systems serve niche requirements where specific shielding, size, or material configurations are necessary. Each type contributes to enhancing safety, regulatory compliance, and process efficiency in high-risk laboratory and production environments.
Dispensing Shielded Cells are applied across radiopharmaceutical manufacturing, chemical synthesis, nuclear medicine, and research laboratories. Radiopharmaceutical manufacturing remains the leading application due to the critical requirement for safe, precise handling of radioactive isotopes. The fastest-growing application is nuclear medicine and clinical diagnostics, fueled by expanding patient diagnostics and therapy programs that require high-throughput, automated dispensing systems. Chemical synthesis applications continue to demand shielded cells for safe manipulation of hazardous or toxic reagents. Research laboratories rely on these systems for experimental flexibility and precision. Across all applications, the emphasis is on operator safety, regulatory compliance, workflow optimization, and integration with automated monitoring or AI-enabled systems, driving consistent adoption across these diverse operational contexts.
Hospitals and specialized nuclear medicine centers are the leading end-users of Dispensing Shielded Cells, largely due to their need for precise and safe administration of radioactive pharmaceuticals in diagnostic and therapeutic applications. The fastest-growing end-user segment is research laboratories within academic and biotech institutions, driven by rising investments in radiopharmaceutical research, clinical trials, and specialized chemical synthesis projects. Industrial manufacturing units also utilize shielded cells for controlled production of hazardous chemicals or isotopes, while contract research organizations increasingly adopt these systems to meet client-specific safety and precision standards. Across all end-users, focus areas include operational efficiency, safety compliance, and compatibility with automation and AI-based monitoring technologies, reflecting a diverse yet interconnected market landscape.
North America accounted for the largest market share at 42% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 7.5% between 2025 and 2032.
North America leads due to the presence of advanced radiopharmaceutical and biotechnology industries, extensive adoption of automated shielded cell systems, and strong regulatory frameworks ensuring safety and compliance. Europe maintains a significant position with high production volumes and integration of AI and robotics in laboratories. Asia-Pacific’s market is expanding rapidly with investments in nuclear medicine, research facilities, and infrastructure modernization. South America and Middle East & Africa are also showing increasing demand due to government support and rising healthcare and industrial applications.
North America accounts for approximately 42% of the global Dispensing Shielded Cell Market. Key industries driving demand include pharmaceutical manufacturing, radiopharmaceutical production, and research laboratories. Notable regulatory changes, such as stricter radiation safety protocols and updated handling standards, have reinforced adoption. Technological advancements include AI-driven monitoring, robotic dispensing systems, and modular designs, improving both operational efficiency and safety. Digital transformation initiatives are enabling seamless integration of shielded cells with laboratory information management systems (LIMS), enhancing workflow optimization and real-time monitoring. The region continues to attract investments in next-generation containment solutions for both research and industrial applications.
Europe holds around 28% of the global market share. Leading European markets include Germany, the UK, and France, where advanced radiopharmaceutical production and biotechnology sectors are concentrated. Regulatory bodies have implemented stringent safety standards and sustainability initiatives, promoting energy-efficient, low-emission shielded cell systems. Adoption of emerging technologies, such as AI monitoring and modular containment solutions, is enhancing operational efficiency and ensuring compliance with EU safety directives. European manufacturers are also focusing on compact, high-density shielding materials that reduce facility space requirements and improve workflow productivity across hospitals, research labs, and production facilities.
Asia-Pacific ranks as the fastest-growing region with a significant market volume. Top consuming countries include China, India, and Japan, which are heavily investing in nuclear medicine, radiopharmaceutical research, and industrial chemical production. Infrastructure trends include the construction of modern containment facilities and adoption of modular shielded cell systems. Regional technological innovation hubs are developing AI-based monitoring systems, remote handling robotics, and high-precision dispensing solutions. The growth in laboratory automation and increasing government support for healthcare and research initiatives are driving adoption across multiple sectors, positioning the region as a critical market for future expansion.
South America accounts for approximately 12% of the global market, with Brazil and Argentina leading adoption. Key drivers include expanding healthcare facilities, radiopharmaceutical production, and research centers. Infrastructure development focuses on modern containment facilities, with energy-efficient designs and integrated safety protocols. Government incentives, trade policies, and partnerships with technology providers encourage investments in advanced shielded cell systems. Regional demand is growing for automated dispensing, radiation containment, and laboratory modernization, providing opportunities for both local and international manufacturers to expand their presence.
Middle East & Africa represent about 8% of the market, with major growth countries including UAE and South Africa. Regional demand trends focus on healthcare, nuclear medicine, and chemical industries requiring high-safety containment systems. Technological modernization includes robotics, AI-based monitoring, and modular construction for shielded cells. Local regulations and trade partnerships support safe handling of radioactive and hazardous materials. Expansion in industrial infrastructure and healthcare facilities, coupled with government initiatives for technological adoption, is driving the uptake of advanced dispensing shielded cell solutions across the region.
United States – 42% Market Share
High production capacity, advanced end-user demand, and strict regulatory compliance drive dominance.
Germany – 15% Market Share
Strong industrial infrastructure and widespread adoption of precision shielded cell systems for research and radiopharmaceutical applications.
The Dispensing Shielded Cell Market is characterized by a highly competitive environment with over 25 active global players operating across North America, Europe, and Asia-Pacific. Market positioning varies, with leading manufacturers emphasizing precision, automation, and containment efficiency to serve pharmaceutical, radiopharmaceutical, and chemical industries. Strategic initiatives include partnerships with research institutions, product launches for modular and automated shielded cells, and acquisitions to expand technological capabilities and geographic reach. Innovation trends focus on AI-integrated monitoring systems, robotic handling, and compact shielding designs that reduce operational footprints while enhancing safety. Companies are increasingly investing in digital transformation, enabling remote operation, real-time monitoring, and seamless integration with laboratory information management systems (LIMS). Competitive differentiation also relies on compliance with evolving regulatory standards and offering customization to meet specific end-user needs. The market is witnessing an accelerated adoption of automated dispensing technologies in research laboratories and industrial production facilities, further intensifying competition and driving continuous technological advancement.
Getinge AB
IBA Radiopharma Solutions
Comecer S.p.A.
LabLogic Systems Ltd.
Eckert & Ziegler AG
Lancer Systems, Inc.
Nordion, Inc.
Biocontainment Technologies Pvt. Ltd.
Synteract, Inc.
R&S Solutions GmbH
Current and emerging technologies in the Dispensing Shielded Cell Market focus on enhancing safety, precision, and operational efficiency. Automated and robotic dispensing systems are increasingly integrated with AI-based monitoring for real-time radiation detection, workflow optimization, and error reduction. Modular shielded cell designs allow rapid installation, flexibility in laboratory layouts, and easier maintenance. Advanced shielding materials such as tungsten composites and lead-free alloys are being adopted to reduce operator exposure while minimizing facility space requirements. Digital control systems enable remote handling, data logging, and integration with laboratory information management systems (LIMS) to streamline operations. Emerging trends include fully automated radiopharmaceutical production lines, precision dosing technologies, and adaptive shielding configurations tailored for high-throughput research applications. These innovations support compliance with strict safety regulations, increase reproducibility in complex chemical or radioactive processes, and reduce manual intervention, positioning manufacturers to meet growing demands in hospitals, research labs, and industrial facilities worldwide.
In February 2024, Comecer S.p.A. launched a new robotic shielded cell system with AI-powered handling, reducing operator exposure by 35% and improving dispensing accuracy for radiopharmaceutical applications.
In September 2023, Getinge AB introduced a modular shielded cell line that allows rapid on-site installation and easy expansion for high-throughput laboratory operations.
In May 2024, Eckert & Ziegler AG unveiled an integrated monitoring system for automated shielded cells, enabling real-time radiation tracking and remote operation across multiple laboratory facilities.
In November 2023, IBA Radiopharma Solutions developed a compact tungsten-composite shielded cell optimized for nuclear medicine research, reducing facility footprint by 22% while maintaining safety standards.
The Dispensing Shielded Cell Market Report offers a comprehensive overview of market segments, regional insights, applications, and technological trends, providing stakeholders with actionable intelligence for strategic planning. The report covers product types such as standard shielded cells, automated robotic cells, modular systems, and customized containment solutions, highlighting their operational applications in radiopharmaceutical production, chemical synthesis, nuclear medicine, and research laboratories. Geographic coverage spans North America, Europe, Asia-Pacific, South America, and the Middle East & Africa, analyzing market volume, infrastructure development, and regional adoption patterns. End-users include hospitals, research laboratories, industrial manufacturing units, and contract research organizations. The report emphasizes technological advancements such as AI integration, robotics, modular design, and advanced shielding materials, while also addressing regulatory compliance, safety standards, and operational efficiency. Additionally, emerging segments like compact and portable shielded cells are examined, alongside trends in digital monitoring and automated dispensing, offering a clear perspective for decision-makers and industry professionals on current and future market opportunities.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2024) | USD 120 Million |
| Market Revenue (2032) | USD 206.2 Million |
| CAGR (2025–2032) | 7.0% |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User Insights
|
| Key Report Deliverables | Revenue Forecast, Growth Drivers & Restraints, Technology Insights, Market Dynamics, Segmentation Analysis, Regional Insights, Competitive Landscape, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Getinge AB, IBA Radiopharma Solutions, Comecer S.p.A., LabLogic Systems Ltd., Eckert & Ziegler AG, Lancer Systems, Inc., Nordion, Inc., Biocontainment Technologies Pvt. Ltd., Synteract, Inc., R&S Solutions GmbH |
| Customization & Pricing | Available on Request (10% Customization is Free) |
