Reports
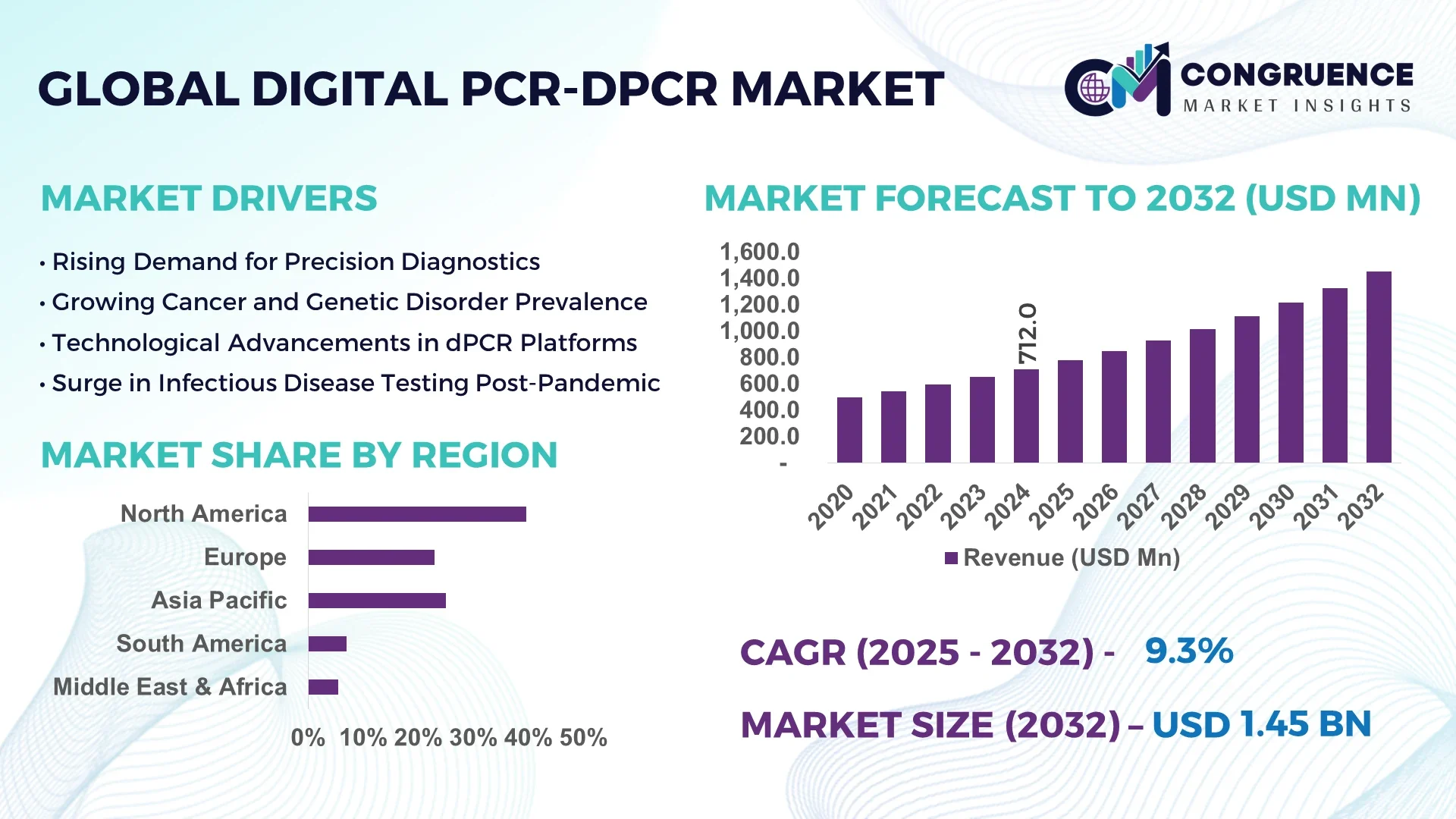
The Global Digital PCR-dPCR Market was valued at USD 712.0 Million in 2024 and is anticipated to reach a value of USD 1,450.2 Million by 2032 expanding at a CAGR of 9.3% between 2025 and 2032.
The United States remains a powerhouse in the Digital PCR-dPCR Market, operating multiple high-throughput production facilities handling over 100,000 assays per year. Major investments—exceeding USD 250 million in 2023—were directed toward advanced droplet and chip-based platforms. U.S.-based clinical laboratories extensively apply dPCR for oncology, infectious disease monitoring, and gene-editing validation, leveraging automated sample partitioning and fluorescence detection systems.
Key sectors within the Digital PCR-dPCR Market include clinical diagnostics, genetic research, infectious disease screening, and pharmaceutical development. Clinical diagnostics account for approximately 40% of platform installations, driven by demand for liquid biopsy assays and transplant viral monitoring. Technological innovation remains pivotal: devices featuring six-color multiplexing and microfluidic sample partitioning now deliver detection efficiencies above 99.5%. Regulatory expectations for assay validation and companion diagnostics have prompted accreditation protocols and GMP-compliant consumables. Environmental and economic pressures—such as the need for benchtop systems with lower power consumption—are pushing portable, low-volume dPCR platforms. Regionally, North America leads in lab adoption, while Europe emphasizes oncology applications, and Asia-Pacific continues to expand in infectious disease surveillance. Emerging trends include edge-capable dPCR systems integrated with cloud analytics and AI-based assay optimization—enabling real-time call thresholds and improved reproducibility across multi-site studies, giving decision-makers enhanced control and predictive insights.
Artificial intelligence is playing a transformative role in the Digital PCR-dPCR Market by optimizing assay design, reducing sample-to-result timelines, and improving data accuracy. AI-driven software now predicts optimal primer and probe combinations based on target sequence analysis, reducing redesign cycles by up to 30%. Embedded algorithms automate droplet classification—differentiating positives and negatives with accuracy improvements of over 99%—reducing manual data review and increasing throughput. Laboratories using AI-enhanced dPCR data interpretation tools report reducing analysis time by 40%, enabling faster clinical decision-making and greater laboratory capacity.
In manufacturing, AI-assisted quality control monitors droplet uniformity, optical channel alignment, and thermal cycling consistency. These systems flag deviations in real-time, enabling corrective calibration actions that reduce fault rates by 25%. During assay development, machine-learning models analyze tens of thousands of droplet fluorescence curves to identify subtle assay failures, improving overall detection sensitivity by up to twofold. Additionally, self-learning software platforms streamline platform calibration workflows, automating baseline shift corrections in as little as 10 minutes.
Supply chain efficiencies are also being realized through AI: predictive component demand modeling ensures labs maintain adequate cartridge and reagent stocks—limiting stock-outs and reducing waste. Taken together, these AI enhancements shift the Digital PCR-dPCR Market from instrument-centric systems to fully intelligent molecular diagnostics platforms, crucial for high-volume clinical and industrial applications.
“In 2024, a European university hospital integrated AI-driven droplet classifiers into its dPCR workflow, increasing sample throughput from 96 to 144 per run and reducing operator review time by 35%.”
The Digital PCR-dPCR Market dynamics are shaped by rapid adoption in clinical diagnostics, rising investment in genomic research, regulatory rigor, and technological innovation. Tool developers are enhancing platform reliability through enhanced multiplexing, faster thermal cycling, and integrated cloud analytics. Demand from personalized medicine and point-of-care testing continues to escalate, influencing instrument design and service models. Evolving lab accreditation standards and reimbursement frameworks shape purchasing decisions, while environmental regulations are prompting greener consumables. The interplay of automation, assay quality, and regulatory compliance defines current competitive and innovation dynamics in the Digital PCR-dPCR Market.
In oncology, demand for liquid biopsy platforms has surged. Hospital laboratories processing ctDNA samples increased assay volume by 45% in 2024. Digital PCR is favored due to its ability to quantify ultra-low-frequency mutations (below 0.1% allele frequency), making it ideal for monitoring minimal residual disease. This driver accelerates instrument adoption in academic centers and mid-tier hospitals around the world.
Assay development for digital PCR requires rigorous validation. Turnkey assays for rare mutation detection need up to 200 validation runs to meet accuracy thresholds. Limited availability of clinical-grade controls and standards delays lab onboarding. Testing centers face extended setup times—often over two months—which restricts adoption despite instrument availability.
The rapid need for pathogen quantification tools in public health has created a significant opportunity. In 2024, U.K. and European wastewater testing initiatives installed portable dPCR units capable of quantifying viral loads in under two hours. These deployments showcase potential use in AMR monitoring and food safety, enabling rapid public health responses with high assay accuracy.
Despite clinical utility, high equipment and consumable costs limit dPCR uptake in low-volume labs. Reagent cartridges, priced between USD 20–30 per run, present significant operating expenses. Combined with instrument costs, this restricts deployment in small regional labs and academic institutions, where patient volume may not justify investment.
Expansion of Multiplexed dPCR Kits: Next-generation dPCR platforms now support six-color detection enabling more than five simultaneous targets per assay. Clinical labs using these kits report reducing repeat runs by 60%, optimizing workflows for comprehensive cancer mutation panels and viral pathogen detection. This directly reduces turnaround time and improves lab efficiency.
Rise of Portable dPCR Platforms for Point-of-Care: Portable droplet and chip digital PCR instruments have been deployed in mobile testing units in remote areas of India and Brazil. These systems, powered by 12‑V battery packs, deliver sample-to-answer results within 90 minutes. Their use in on-site diagnostics for HIV and TB demonstrates strong field applicability.
Integration of Cloud-Based Data Analytics: Several commercial dPCR systems now offer cloud-enabled data pipelines that allow multi-site labs to share test results securely. Centralized dashboards compare performance metrics in real-time—such as positive droplet rates and confidence intervals—enabling centralized quality control and lab benchmarking across regions.
Introduction of Single-Cell dPCR Solutions: Digital PCR instruments are now being adapted for single-cell assays in immuno-oncology applications. Technologies enabling partitioning at sub-nanoliter volumes allow quantification of gene expression in minimal samples. Pilot academic use reports quantifying target transcripts from under 500 cells, supporting advanced research and contract development projects.
The Digital PCR-dPCR Market is segmented based on type, application, and end-user. Each segment offers a detailed view into how this technology is being adopted across diverse scientific and clinical landscapes. Types of digital PCR platforms include droplet digital PCR, chip-based digital PCR, and BEAMing digital PCR, each tailored for varying throughput and assay requirements. Applications span oncology, infectious disease detection, genetic research, and forensic science. End-users include academic institutions, hospitals, clinical laboratories, and pharmaceutical companies, all of which contribute uniquely to the market’s growth. Understanding these segmentation parameters is crucial for industry stakeholders aiming to identify demand hotspots, develop targeted product portfolios, and make informed investment decisions. Differentiated product capabilities, regulatory compliance standards, and workflow compatibility are key factors shaping market preference within each segment. The expansion of high-sensitivity diagnostics and precision medicine is further intensifying the adoption of specific types and applications, positioning segmentation as a core element of market strategy.
The type segment in the Digital PCR-dPCR Market is categorized into droplet digital PCR (ddPCR), chip-based digital PCR, and BEAMing digital PCR. Among these, droplet digital PCR holds the leading position due to its high sensitivity, broad dynamic range, and ability to partition samples into tens of thousands of droplets—ensuring statistically robust quantification. Its utility in mutation detection and gene expression analysis across oncology and virology has contributed to its widespread adoption in hospital labs and research institutions.
Chip-based digital PCR is currently the fastest-growing segment, particularly favored in multiplexed clinical assays. With automation-friendly formats and ease of use, chip-based platforms reduce cross-contamination risks and integrate well into high-throughput diagnostic workflows. Advancements in chip miniaturization and optical readout systems are fueling this growth.
BEAMing digital PCR, although more niche, is gaining traction for its use in liquid biopsy and circulating tumor DNA analysis. Its magnetic bead-based partitioning mechanism offers excellent precision for rare mutation detection in oncology, especially within pharmaceutical development and academic research settings.
Applications of digital PCR-dPCR technology span several vital domains. Oncology diagnostics leads the application landscape, driven by the need for ultra-sensitive detection of mutations and copy number variations in circulating tumor DNA. dPCR is a key enabler in non-invasive cancer monitoring, with major cancer centers using it for real-time tracking of minimal residual disease and treatment response.
Infectious disease detection is emerging as the fastest-growing application. Its rise is fueled by the growing demand for accurate, rapid, and multiplex pathogen detection—particularly during public health emergencies. dPCR has shown superior performance in detecting low-abundance viral and bacterial DNA/RNA, making it an indispensable tool for hospital infection control and outbreak monitoring.
Other significant application areas include genetic disease research, where dPCR supports gene editing validation and rare allele quantification, and prenatal diagnostics, where it provides non-invasive testing options. Environmental testing and food safety also represent niche but growing application spaces due to dPCR’s precision and sensitivity in detecting microbial contamination.
The largest end-user segment in the Digital PCR-dPCR Market is clinical diagnostic laboratories. These facilities rely heavily on dPCR for high-accuracy quantification of genetic targets in applications like liquid biopsy, viral load monitoring, and transplant diagnostics. Labs equipped with dPCR platforms report increased reproducibility and reduced false positives compared to qPCR-based systems, making it the method of choice for critical clinical applications.
Pharmaceutical and biotechnology companies represent the fastest-growing end-user segment. Their growing reliance on digital PCR for drug development, biomarker validation, and clinical trial monitoring is accelerating platform installations. The technology’s capacity for absolute quantification without the need for standard curves is especially valuable in precision drug development.
Other end-users such as academic and research institutes remain important contributors. These organizations employ dPCR to advance fundamental genomic research and validate CRISPR gene editing experiments. Public health agencies and food safety labs also deploy dPCR tools for contamination surveillance and disease outbreak management, underscoring its broad cross-sector appeal.
North America accounted for the largest market share at 39.6% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 11.2% between 2025 and 2032.

North America’s dominance is underpinned by advanced healthcare infrastructure, significant investments in precision medicine, and widespread adoption of high-throughput genomic testing. Meanwhile, the Asia-Pacific region is witnessing rapid adoption of digital PCR technologies due to expanding clinical diagnostic networks, growing research capabilities, and government-led genomics initiatives. The market in Europe remains strong, with a focus on regulatory compliance and early disease detection programs, while emerging regions such as South America and the Middle East & Africa show promising uptake due to improving laboratory capabilities and rising awareness of molecular diagnostics. Market players are increasingly localizing production, expanding distribution networks, and forging research partnerships to capitalize on the growth dynamics unique to each region.
North America held approximately 39.6% of the global market share in 2024, maintaining its position as the primary hub for digital PCR technology deployment. The region's market is fueled by the high prevalence of chronic diseases, robust funding for life sciences research, and a large base of clinical laboratories integrating dPCR for early disease detection. The U.S. drives demand with consistent technological advancements in cancer diagnostics and infectious disease monitoring. Additionally, regulatory reforms such as the FDA’s support for next-generation molecular diagnostics are accelerating innovation. Integration of digital PCR platforms with AI-enabled data analytics tools is further strengthening adoption across hospitals and pharmaceutical companies.
Europe continues to be a vital contributor to the Digital PCR-dPCR Market, accounting for nearly 27.1% of the total volume in 2024. Germany, the UK, and France remain key players, driven by their mature healthcare ecosystems and emphasis on research-based innovation. National healthcare frameworks encourage the integration of advanced diagnostic technologies like dPCR into routine clinical workflows. Regulatory bodies, including the European Medicines Agency (EMA), are streamlining approval processes for molecular diagnostics, making way for faster market entry. European biotech companies are increasingly focusing on digital PCR to support applications in personalized medicine, rare disease diagnostics, and pharmaceutical development.
The Asia-Pacific region ranked second in market volume and is experiencing the fastest growth trajectory. China, India, and Japan are the leading consumers of digital PCR platforms, supported by large patient populations, an expanding base of clinical labs, and increased government funding for genomics. In China, the national genomics project is accelerating dPCR technology adoption in oncology and infectious disease surveillance. India’s focus on building genetic testing infrastructure and Japan’s pharmaceutical innovations are also contributing to the surge in demand. Regional tech hubs are enabling the emergence of AI-integrated dPCR platforms and cloud-connected diagnostic systems, enhancing operational efficiency across clinical workflows.
South America is steadily expanding its footprint in the Digital PCR-dPCR Market. Brazil and Argentina are the top contributing nations, driven by improvements in public healthcare infrastructure and rising investments in diagnostic technologies. Brazil leads in market share due to government-backed initiatives for expanding access to molecular testing, especially in oncology and infectious disease sectors. Argentina has seen recent investments in life sciences R&D, improving accessibility to cutting-edge PCR equipment in research labs and hospitals. Trade incentives and regional partnerships are fostering growth, especially in clinical genomics and academic collaborations.
In the Middle East & Africa, the Digital PCR-dPCR Market is gaining traction, especially in countries like UAE and South Africa. The demand is driven by the need for accurate diagnostics in infectious disease control, including tuberculosis and viral load monitoring. UAE’s rapid adoption of genomics-based healthcare strategies and South Africa’s efforts to strengthen molecular diagnostic capabilities are playing key roles. Investment in smart healthcare systems and regulatory modernization is facilitating the entry of advanced diagnostic equipment. Additionally, regional collaborations with global biotech companies are enhancing training and accessibility in both public and private healthcare sectors.
United States - 31.2% Market Share
Strong end-user demand in clinical diagnostics and robust investments in life sciences R&D fuel market dominance.
China - 18.4% Market Share
High production capacity and large-scale adoption in precision medicine and infectious disease testing drive leadership.
The Digital PCR-dPCR Market is characterized by a moderately consolidated competitive landscape, with over 30 active global and regional players contributing to innovation and market growth. Leading companies focus on expanding their portfolios through technological advancements in droplet-based, chip-based, and BEAMing dPCR systems. Strategic partnerships, frequent product launches, and acquisitions are common approaches to gaining market presence. For instance, collaborations between diagnostic firms and research institutions are accelerating the development of disease-specific assays for oncology, virology, and prenatal testing. Additionally, companies are investing in automation-enabled platforms with higher sensitivity and scalability, catering to both clinical diagnostics and research applications. The competition is particularly intense in North America and Europe, where product differentiation and intellectual property play crucial roles. In emerging regions, cost-effective dPCR systems tailored for decentralized testing environments are gaining traction. Companies are also leveraging cloud integration and AI-driven analytics to enhance the utility and adoption of their dPCR offerings.
Bio-Rad Laboratories, Inc.
Thermo Fisher Scientific, Inc.
QIAGEN N.V.
JN Medsys
RainDance Technologies, Inc.
Sysmex Corporation
Stilla Technologies
Combinati, Inc.
Fluidigm Corporation
Thermogenesis Holdings, Inc.
Danaher Corporation
Biomérieux SA
Avance Biosciences Inc.
Takara Bio Inc.
Technological advancements continue to shape the future of the Digital PCR-dPCR Market, driving improvements in accuracy, throughput, and ease of use. One of the most transformative developments is the emergence of droplet digital PCR (ddPCR), which partitions samples into thousands of nanoliter-sized droplets, allowing absolute quantification without the need for reference standards. This technology is increasingly adopted in oncology for mutation detection and minimal residual disease monitoring due to its high sensitivity.
Another key innovation is the integration of chip-based systems, which enable parallel processing of multiple reactions on a single microfluidic chip. These platforms are popular in pharmaceutical R&D and infectious disease diagnostics, where time-efficient and cost-effective workflows are essential.
Advancements in multiplexing capabilities are also revolutionizing assay development, enabling the simultaneous detection of multiple genetic targets. This is particularly valuable in pathogen detection and genetic disorder screening. Furthermore, the incorporation of AI and machine learning into digital PCR software enhances data analysis, reducing errors and enabling real-time decision-making.
Portability and miniaturization of dPCR platforms are also expanding market reach, especially in remote or resource-limited settings. Innovations in reagent chemistry, such as enzyme stabilization and lyophilization, are further extending the shelf-life and usability of kits, making them viable for global distribution and decentralized testing.
In April 2024, QIAGEN launched a new dPCR assay panel for detecting emerging SARS-CoV-2 variants with improved accuracy and a turnaround time of under 2 hours, addressing the demand for rapid pandemic surveillance tools.
In December 2023, Bio-Rad Laboratories introduced its next-generation QX600™ Droplet Digital PCR System, offering enhanced multiplexing with up to six-color detection, improving research productivity in oncology and infectious disease diagnostics.
In August 2024, Stilla Technologies expanded its Naica® system portfolio with an AI-powered image analysis module, enabling higher resolution droplet imaging and better precision in low-abundance target detection for rare mutation analysis.
In March 2023, Thermo Fisher Scientific announced the development of an ultra-high-throughput dPCR platform for non-invasive prenatal testing (NIPT), designed to process over 5,000 samples per day, boosting efficiency in large-scale clinical settings.
The Digital PCR-dPCR Market Report provides a comprehensive analysis of the global landscape, encompassing a detailed segmentation by type, application, and end-user. The report evaluates various technologies including droplet-based, chip-based, and BEAMing digital PCR systems. Applications span across oncology, infectious disease diagnostics, genetic testing, pharmacogenomics, and environmental monitoring. It also profiles industry usage across clinical laboratories, academic research institutions, pharmaceutical companies, and biotechnology firms.
Geographically, the report covers market dynamics across North America, Europe, Asia-Pacific, South America, and the Middle East & Africa, examining regional drivers, infrastructure readiness, and regulatory trends. It also highlights emerging countries with growing investments in molecular diagnostics.
Technological insights focus on automation, miniaturization, and AI-integration, while also addressing market disruptors such as reagent innovations and connected diagnostic ecosystems. The report identifies strategic opportunities in personalized medicine, decentralized healthcare, and real-time monitoring applications, helping stakeholders understand the competitive and innovation-driven direction of the market.
This strategic overview equips decision-makers, analysts, and industry participants with actionable intelligence, aiding in market entry planning, investment assessment, and long-term business development.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2024) | USD 712.0 Million |
| Market Revenue (2032) | USD 1,450.2 Million |
| CAGR (2025–2032) | 9.3% |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User
|
| Key Report Deliverables | Revenue Forecast, Growth Drivers & Restraints, Technology Insights, Market Dynamics, Segmentation Analysis, Regional Insights, Competitive Landscape, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Bio-Rad Laboratories, Inc., Thermo Fisher Scientific, Inc., QIAGEN N.V., JN Medsys, RainDance Technologies, Inc., Sysmex Corporation, Stilla Technologies, Combinati, Inc., Fluidigm Corporation, Thermogenesis Holdings, Inc., Danaher Corporation, Biomérieux SA, Avance Biosciences Inc., Takara Bio Inc. |
| Customization & Pricing | Available on Request (10% Customization is Free) |
