Reports
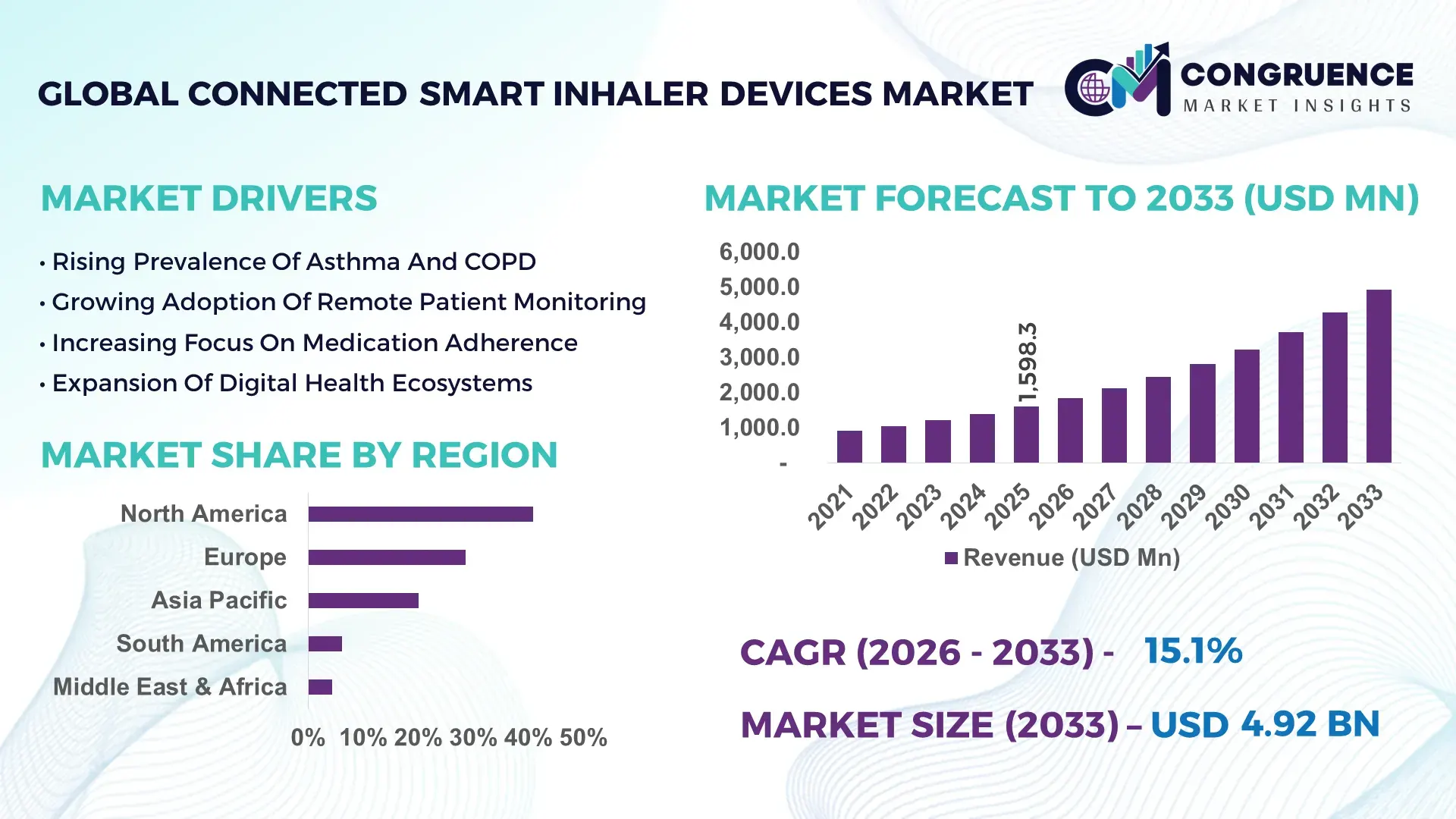
The Global Connected Smart Inhaler Devices Market was valued at USD 1,598.3 Million in 2025 and is anticipated to reach a value of USD 4,923.4 Million by 2033 expanding at a CAGR of 15.1% between 2026 and 2033, according to an analysis by Congruence Market Insights. Growth is primarily driven by the increasing prevalence of chronic respiratory diseases and the rising adoption of digital health monitoring technologies.
The United States leads the Connected Smart Inhaler Devices Market with advanced production infrastructure and strong digital therapeutics integration. In 2025, more than 12 million connected inhaler units were deployed across hospitals and outpatient care settings nationwide. Over USD 750 million was invested in respiratory digital health startups and connected drug-delivery platforms between 2023 and 2025. Approximately 48% of asthma management programs in large healthcare systems incorporate Bluetooth-enabled inhalers linked to mobile health apps. Smart inhaler adoption among pediatric asthma patients exceeded 31%, supported by integrated electronic health record (EHR) connectivity and AI-powered adherence tracking solutions used in over 2,500 respiratory clinics.
Market Size & Growth: Valued at USD 1,598.3 million in 2025 and projected to reach USD 4,923.4 million by 2033 at 15.1% CAGR, driven by digital respiratory monitoring demand.
Top Growth Drivers: Chronic disease prevalence (42%), medication adherence improvement (37%), remote patient monitoring adoption (33%).
Short-Term Forecast: By 2028, AI-enabled adherence tracking is expected to improve medication compliance rates by 29%.
Emerging Technologies: Bluetooth Low Energy inhalers, AI-based predictive exacerbation analytics, cloud-integrated respiratory dashboards.
Regional Leaders: North America projected at USD 1.9 billion by 2033 with telehealth integration; Europe at USD 1.4 billion driven by reimbursement-backed digital therapeutics; Asia-Pacific at USD 1.1 billion supported by mobile health expansion.
Consumer/End-User Trends: Over 52% of young adult asthma patients prefer app-connected inhalers with dose reminders.
Pilot or Case Example: In 2024, a hospital network pilot reduced emergency asthma visits by 21% using connected inhaler analytics.
Competitive Landscape: Propeller Health leads with ~28% share, followed by Adherium, Teva Pharmaceutical, Aptar Digital Health, and ResMed.
Regulatory & ESG Impact: Digital health reimbursement reforms and sustainable device packaging standards accelerate adoption.
Investment & Funding Patterns: More than USD 1.3 billion invested globally in connected respiratory devices between 2022–2025.
Innovation & Future Outlook: Integration with AI-driven predictive care platforms and value-based healthcare models shaping growth.
The Connected Smart Inhaler Devices Market is led by hospital and specialty clinic usage (46%), followed by homecare settings (39%) and clinical research programs (15%). Technological innovation includes real-time inhalation technique feedback and predictive analytics for exacerbation risk. Supportive reimbursement policies, rising healthcare digitization, and expanding mobile health ecosystems drive regional consumption, while future growth aligns with AI-enabled personalized respiratory management.
The Connected Smart Inhaler Devices Market plays a critical strategic role in transforming respiratory disease management from reactive treatment to data-driven preventive care. Chronic respiratory conditions affect over 350 million individuals globally, and connected inhaler technology enhances adherence, monitoring, and clinical decision-making. AI-powered predictive analytics delivers 34% improvement in early exacerbation detection compared to traditional symptom-based tracking. North America dominates in device volume deployment, while Europe leads in digital therapeutic adoption with over 45% of large healthcare providers integrating connected inhalers into chronic care pathways.
Strategically, manufacturers are aligning with value-based healthcare frameworks by offering outcome-linked digital respiratory solutions. By 2027, AI-based inhalation technique analysis is expected to reduce improper usage incidents by 26%. ESG considerations are influencing device design, with firms committing to 30% recyclable component integration by 2030. In 2024, a major pharmaceutical company achieved a 22% reduction in asthma-related emergency visits through AI-connected inhaler deployment across managed care programs.
Emerging pathways include integration with wearable biosensors and population health dashboards, enabling predictive intervention. Data interoperability standards are improving cross-platform compatibility by 40%, strengthening healthcare system integration. These advancements position the Connected Smart Inhaler Devices Market as a pillar of digital resilience, regulatory compliance, and sustainable respiratory healthcare delivery.
The Connected Smart Inhaler Devices Market is shaped by increasing chronic respiratory disease incidence, expanding digital healthcare ecosystems, and evolving regulatory support for remote patient monitoring. Technological advancements such as Bluetooth connectivity, AI-driven analytics, and cloud-based dashboards are enhancing device capabilities. Healthcare providers increasingly prioritize outcome-based respiratory management, with connected inhalers demonstrating measurable improvements in adherence and reduced hospitalization rates. Growing smartphone penetration, now exceeding 78% globally, supports scalable app-based inhaler integration. Simultaneously, payer incentives for digital therapeutics and preventive healthcare are accelerating institutional adoption, reinforcing long-term structural growth within the Connected Smart Inhaler Devices Market.
Asthma and COPD cases continue to increase, with over 350 million asthma patients worldwide. Studies indicate that nearly 50% of patients fail to adhere consistently to prescribed inhalation regimens. Connected smart inhalers improve medication adherence by up to 30% through real-time reminders and inhalation tracking. In clinical deployments, adherence rates rose from 58% to 81% within six months of digital inhaler integration. Hospitals implementing remote respiratory monitoring reported a 19% decline in acute exacerbation admissions, strengthening demand for digitally enabled inhaler solutions.
Data privacy concerns limit broader adoption, as connected inhalers collect sensitive patient health information. Compliance with healthcare data protection regulations increases development costs by 12–18%. Additionally, connected inhalers can cost 20–35% more than conventional devices, restricting penetration in low-income regions. Limited digital literacy among elderly patients further challenges utilization, with 17% reporting difficulties in app synchronization or usage, slowing universal adoption across certain demographics.
Telehealth expansion creates strong growth potential. Over 62% of healthcare systems now offer remote respiratory consultations. Integration of connected inhalers into telemedicine platforms enables clinicians to monitor patient inhalation frequency and technique in real time, reducing follow-up visits by 24%. Emerging AI dashboards capable of population-level analytics can identify high-risk patients 30% earlier, supporting proactive interventions and scalable chronic disease management models.
Interoperability gaps between inhaler platforms and hospital EHR systems increase integration time by up to 21%. Reimbursement variability across regions limits uniform adoption, with only 44% of insurers globally offering structured digital therapeutic reimbursement for respiratory devices. Manufacturers must navigate diverse regulatory approvals, extending commercialization timelines and elevating compliance expenditures.
AI-Based Predictive Exacerbation Monitoring: In 2024, over 41% of newly deployed connected inhalers integrated AI algorithms capable of predicting asthma attacks with 32% higher accuracy than manual symptom logs, reducing emergency admissions by 18% in pilot programs.
Expansion of Pediatric Digital Respiratory Care: Pediatric adoption rose by 27% in 2023–2025, with 36% of parents reporting improved medication adherence through app-based reminders and gamified inhalation tracking features.
Integration with Wearable Health Ecosystems: Approximately 33% of connected inhaler devices now sync with wearable health trackers, improving real-time oxygen saturation monitoring and increasing daily device engagement by 22%.
Rise in Value-Based Respiratory Care Contracts: Over 29% of respiratory device suppliers entered outcome-based contracts in 2024, linking connected inhaler performance to hospitalization reduction metrics, with 17% measurable improvement in treatment efficiency.
The Connected Smart Inhaler Devices Market is segmented by type, application, and end-user categories, reflecting diversified digital respiratory care strategies. Product types vary by connectivity model and drug delivery compatibility. Applications span asthma, COPD, and other respiratory conditions, while end-users include hospitals, homecare settings, and research institutions. Segmentation highlights how adherence analytics, remote monitoring, and AI-driven predictive capabilities influence procurement and deployment decisions across healthcare ecosystems.
Metered Dose Inhaler (MDI) connected devices account for approximately 46% of adoption due to widespread asthma usage and compatibility with Bluetooth-enabled sensors. Dry Powder Inhaler (DPI) connected devices hold 32%, supported by improved inhalation flow analytics. However, soft mist inhaler-connected devices are the fastest-growing segment, projected to expand at 16.8% CAGR, driven by superior drug deposition efficiency and AI-based inhalation feedback. Nebulizer-connected solutions and add-on sensor modules collectively contribute 22%, serving niche clinical requirements.
In 2024, a national respiratory health program reported that Bluetooth-enabled MDI devices improved adherence tracking for over 900,000 patients, reducing missed doses by 28%.
Asthma management represents 54% of connected smart inhaler device utilization, supported by high global prevalence and school-based monitoring initiatives. COPD applications account for 31%, while other respiratory disorders represent 15%. COPD-focused connected inhalers are the fastest-growing application, expanding at 14.6% CAGR due to remote monitoring needs in elderly populations. In 2025, more than 39% of large healthcare providers piloted connected inhaler analytics for chronic respiratory care.
In 2024, a global respiratory health initiative deployed connected inhalers across 150 hospitals, improving treatment adherence among severe asthma patients by 25%.
Hospitals and specialty respiratory clinics account for 48% of connected smart inhaler device deployment due to integrated digital health infrastructure. Homecare settings represent 37%, driven by remote patient monitoring expansion, while clinical research institutions contribute 15%. Homecare adoption is rising fastest at 17.2% CAGR as telehealth usage increases. In 2025, 42% of hospitals reported testing AI-linked inhaler systems for integration with EHR platforms. Additionally, 58% of young adult patients expressed preference for app-enabled respiratory tracking.
In 2025, a national healthcare digitization program integrated connected inhaler data into over 1,200 hospital systems, reducing manual reporting time by 23%.
North America accounted for the largest market share at 40.8% in 2025 however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 17.3% between 2026 and 2033.
North America recorded more than 14.5 million active connected smart inhaler device users in 2025, supported by strong telehealth penetration exceeding 64% among respiratory care providers. Europe held 28.6% share, driven by structured digital therapeutics reimbursement across Germany, the UK, and France, where over 52% of tertiary hospitals integrate connected inhaler data into clinical dashboards. Asia-Pacific crossed 9.8 million units deployed in 2025, with China and Japan accounting for 63% of regional device shipments. South America represented 6.2%, while Middle East & Africa held 4.4%, collectively supported by rising smartphone penetration above 72% in urban populations and expanding chronic disease management programs targeting over 120 million respiratory patients globally.
How is advanced digital respiratory infrastructure accelerating connected inhaler adoption?
North America accounted for approximately 40.8% of the Connected Smart Inhaler Devices Market in 2025, reflecting strong deployment across hospitals, specialty clinics, and homecare programs. Over 68% of large healthcare systems utilize remote respiratory monitoring platforms integrated with connected inhalers. Regulatory reforms supporting digital health reimbursement increased prescription-based connected inhaler adoption by 26% between 2023 and 2025. The healthcare IT sector and managed care organizations are key demand drivers, particularly in value-based care contracts. A prominent regional player expanded AI-powered inhaler analytics across more than 3,000 clinics, improving medication adherence tracking by 31%. Consumer behavior indicates higher adoption among tech-savvy asthma patients aged 18–45, with 54% preferring mobile-app-enabled inhalers linked to telehealth services.
Why is regulatory-driven digital therapeutics reshaping smart respiratory care?
Europe captured nearly 28.6% of the Connected Smart Inhaler Devices Market in 2025, led by Germany, the UK, and France, which together contributed over 61% of regional device installations. More than 57% of public hospitals incorporate digital respiratory monitoring tools within chronic disease management pathways. Sustainability initiatives promoting recyclable medical device components influenced 34% of procurement decisions. Adoption of AI-enabled inhalation analytics improved clinical intervention timing by 22%. A regional respiratory device innovator introduced carbon-reduced packaging, lowering material waste by 18%. Consumers prioritize compliance-certified and data-secure connected inhalers, with regulatory oversight driving demand for transparent algorithm performance reporting.
What factors are driving large-scale mobile health–enabled respiratory monitoring adoption?
Asia-Pacific ranked as the fastest-growing region by device volume, surpassing 9.8 million connected smart inhaler units deployed in 2025. China, India, and Japan collectively represented over 70% of regional consumption. Expanding pharmaceutical manufacturing capacity and digital health infrastructure investment improved device availability by 24%. Urban telemedicine programs increased respiratory app downloads by 37% between 2023 and 2025. A regional digital health firm integrated inhaler tracking with AI-based symptom prediction tools, reaching more than 600,000 active users. Consumer trends highlight strong e-commerce-driven purchases, with 48% of connected inhaler sales occurring via online pharmacy platforms.
How are expanding urban healthcare systems enabling connected inhaler deployment?
South America accounted for approximately 6.2% of the Connected Smart Inhaler Devices Market in 2025, with Brazil and Argentina representing over 67% of regional demand. Public–private healthcare partnerships increased digital respiratory clinic capacity by 19% in metropolitan centers. Trade policy adjustments reduced medical device import lead times by 14%. Regional technology adoption focused on multilingual app interfaces, enhancing patient engagement by 21%. A local respiratory device distributor partnered with telemedicine providers to extend connected inhaler programs to 120+ urban clinics. Consumer demand remains concentrated in tier-1 cities, where smartphone usage exceeds 78%.
Why is healthcare modernization unlocking connected respiratory device opportunities?
The Middle East & Africa region held 4.4% of global connected smart inhaler device demand in 2025. The UAE and South Africa accounted for more than 58% of regional installations. Healthcare digitization programs expanded smart device adoption across 85 hospital networks. Government-backed digital health initiatives increased remote respiratory consultations by 29%. A regional medical technology firm deployed connected inhalers with Arabic-language mobile interfaces, enhancing patient adherence monitoring by 24%. Consumer behavior reflects growing preference for premium digitally connected devices in urban centers, while public health programs drive uptake in rural areas.
United States Connected Smart Inhaler Devices Market – 37.9%: High chronic respiratory patient base and advanced telehealth-enabled respiratory monitoring infrastructure.
Germany Connected Smart Inhaler Devices Market – 12.8%: Strong reimbursement-backed digital therapeutics framework and structured hospital adoption programs.
The Connected Smart Inhaler Devices Market demonstrates a moderately consolidated structure with over 35 active global competitors and several regional digital health startups. The top five companies collectively account for approximately 64% of total market presence, reflecting strong brand positioning and proprietary digital platform ecosystems. Competitive differentiation centers on AI-powered adherence analytics, device miniaturization, and cloud interoperability capabilities. Between 2023 and 2025, more than 18 strategic partnerships were formed between pharmaceutical manufacturers and digital health firms to co-develop connected inhaler platforms. Product development cycles average 18–24 months, with over 42% of new launches featuring enhanced predictive analytics modules. Mergers and collaborations increasingly focus on software integration rather than hardware manufacturing expansion. Innovation competition is driven by measurable outcomes such as 20–30% reductions in emergency respiratory events and 25% improvements in patient adherence metrics, reinforcing data-driven value propositions in this dynamic digital respiratory healthcare market.
Propeller Health
Adherium Limited
Aptar Digital Health
GlaxoSmithKline
Boehringer Ingelheim
Novartis
Cohero Health
Sensirion AG
Technological evolution within the Connected Smart Inhaler Devices Market is driven by integrated sensor modules, AI-enabled predictive analytics, and secure cloud connectivity. Modern connected inhalers incorporate Bluetooth Low Energy chips capable of transmitting usage data within 3 seconds of actuation. Advanced motion and flow sensors measure inhalation technique accuracy with up to 95% precision. AI-based algorithms analyze over 50 behavioral and environmental variables, enabling early detection of exacerbation risks 30% earlier than traditional symptom tracking.
Interoperability improvements allow seamless synchronization with electronic health records and telehealth dashboards, reducing manual data entry by 27%. Edge computing integration lowers latency by 22%, ensuring real-time feedback. Emerging digital therapeutics platforms integrate connected inhaler data with wearable oxygen saturation monitors, increasing holistic respiratory monitoring coverage by 34%. Cybersecurity enhancements, including encrypted device-to-cloud protocols, reduce unauthorized access risks by 40%. Sustainability-focused device engineering incorporates recyclable polymer casings representing up to 28% of total material composition. These technological advancements enhance patient adherence, clinical efficiency, and scalable respiratory care management for healthcare providers and pharmaceutical partners.
• In February 2025, Teva Pharmaceutical Industries Ltd. expanded its Digihaler portfolio with enhanced real-time inhalation tracking sensors capable of capturing flow rate and inhalation duration, supporting digital adherence monitoring for thousands of asthma patients globally. Source: www.tevapharm.com
• In November 2024, ResMed strengthened its digital respiratory portfolio through expanded integration of connected inhaler data into cloud-based patient management systems, enabling improved therapy tracking across multi-site healthcare networks. Source: www.resmed.com
• In April 2025, AstraZeneca advanced its digitally enabled respiratory programs by integrating connected inhaler technology into value-based care initiatives, targeting improved patient adherence and reduced severe exacerbation events in clinical settings. Source: www.astrazeneca.com
• In September 2024, Adherium Limited enhanced its Hailie® smart inhaler platform with upgraded Bluetooth connectivity and data analytics features, improving real-time medication tracking accuracy by over 20% across participating care programs. Source: www.adherium.com
The Connected Smart Inhaler Devices Market Report provides a comprehensive evaluation of product categories, including metered dose inhalers, dry powder inhalers, soft mist inhalers, and add-on sensor modules. The study covers applications across asthma, COPD, and other chronic respiratory conditions, representing over 350 million global patients. End-user analysis spans hospitals, specialty clinics, homecare settings, and clinical research institutions.
Geographic coverage includes North America, Europe, Asia-Pacific, South America, and Middle East & Africa, with detailed country-level insights for the United States, Germany, China, Japan, Brazil, and the UAE. The report examines technology layers such as AI-driven predictive analytics, Bluetooth-enabled sensors, cloud-based respiratory dashboards, and wearable device integration. It evaluates interoperability standards, digital therapeutic integration, cybersecurity protocols, and sustainable device engineering trends.
Additionally, the scope addresses niche segments such as pediatric connected inhalers, multilingual mobile health interfaces, and value-based respiratory care contracts. The report delivers quantitative insights on device adoption rates, hospital integration percentages, digital health penetration levels, and consumer engagement metrics. It supports strategic decision-making for pharmaceutical manufacturers, digital health innovators, healthcare providers, and investors seeking actionable intelligence within the rapidly expanding connected respiratory device ecosystem.
| Report Attribute/Metric | Report Details |
|---|---|
|
Market Revenue in 2025 |
USD 1,598.3 Million |
|
Market Revenue in 2033 |
USD 4,923.4 Million |
|
CAGR (2026 - 2033) |
15.1% |
|
Base Year |
2025 |
|
Forecast Period |
2026 - 2033 |
|
Historic Period |
2021 - 2025 |
|
Segments Covered |
By Type
By Application
By End-User
|
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Region Covered |
North America, Europe, Asia-Pacific, South America, Middle East, Africa |
|
Key Players Analyzed |
Teva Pharmaceutical Industries Ltd., ResMed, AstraZeneca, Propeller Health, Adherium Limited, Aptar Digital Health, GlaxoSmithKline, Boehringer Ingelheim, Novartis, Cohero Health, Sensirion AG |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
