Reports
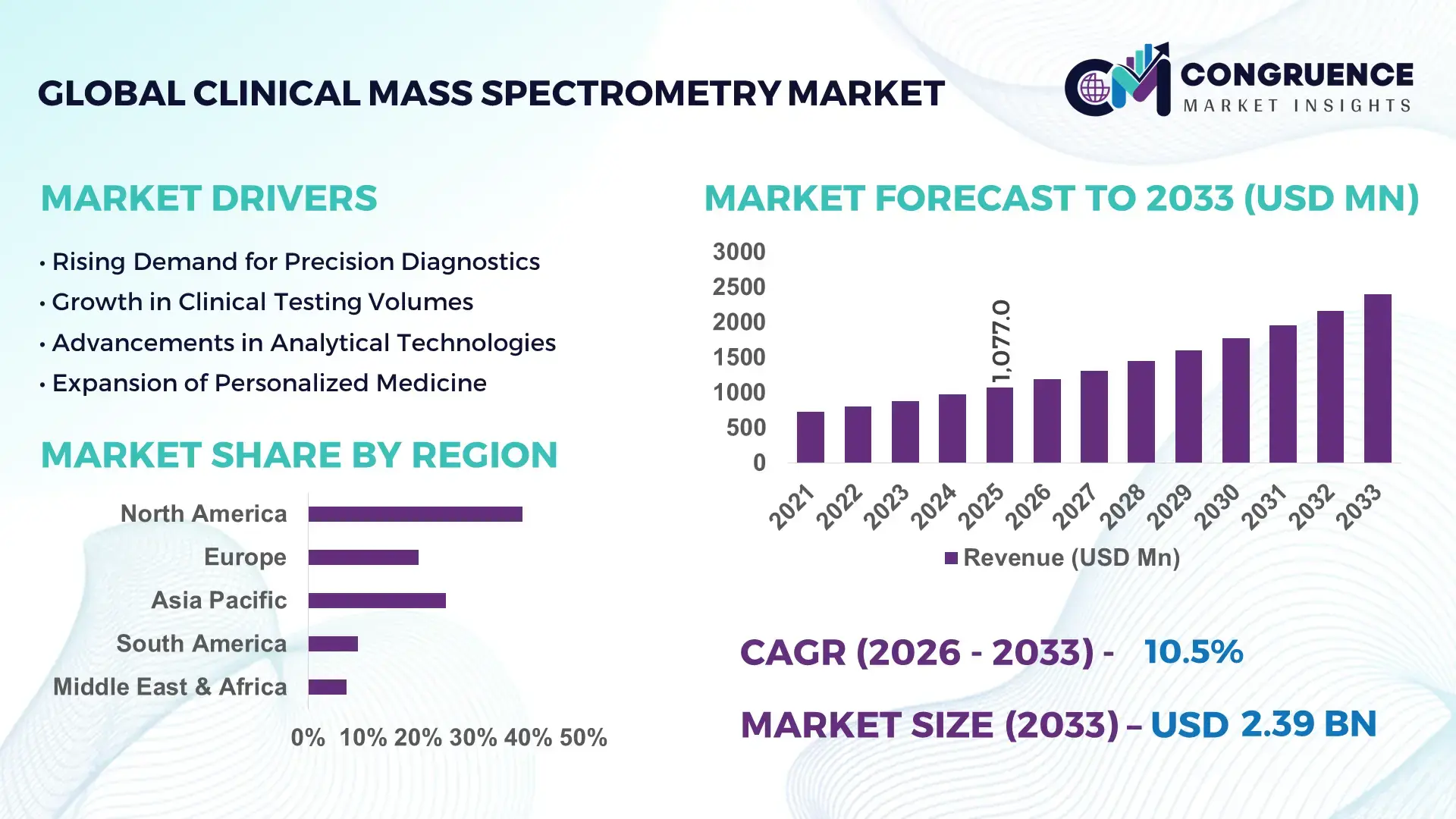
The Global Clinical Mass Spectrometry Market was valued at USD 1077 Million in 2025 and is anticipated to reach a value of USD 2393.94 Million by 2033 expanding at a CAGR of 10.5% between 2026 and 2033. This growth is driven by rapid adoption of advanced diagnostic technologies in clinical laboratories and increasing demand for precise disease detection and personalized medicine.
In the United States, extensive adoption of clinical mass spectrometry systems across hospitals, diagnostic labs, and research institutions underscores its leading role in this market. Over 74% of reference laboratories in the U.S. now utilize mass spectrometry for drug monitoring and toxicology, and a growing number of university medical centers have integrated tandem mass spectrometers in clinical departments. Investments in laboratory automation and AI-enabled spectrometry technologies have risen significantly, with approximately 68% of academic hospitals deploying high‑performance MS systems for hormone profiling, metabolic analysis, and newborn screening. U.S. clinical mass spectrometry installations continue to expand, reflecting strong institutional commitment to advanced diagnostics, R&D, and clinical research applications.
Market Size & Growth: Valued at USD 1077 Million in 2025 and projected to reach USD 2393.94 Million by 2033 at a CAGR of 10.5%, supported by rising precision diagnostics and clinical research demand.
Top Growth Drivers: Increased adoption in clinical labs (+45%), expansion of precision medicine initiatives (+38%), rising integration with AI and automation (+33%).
Short-Term Forecast: By 2028, system throughput and efficiency projected to improve cost‑per‑test by ~18% in high‑volume clinical settings.
Emerging Technologies: AI‑assisted spectral analysis, miniaturized portable MS units, and automated sample prep platforms.
Regional Leaders: North America (~USD 950M by 2033), Europe (~USD 710M by 2033), Asia‑Pacific (~USD 550M by 2033) with rising healthcare modernization; unique trend: strong clinical R&D growth in Asia‑Pacific.
Consumer/End‑User Trends: Hospitals and diagnostic centers increasingly adopt LC‑MS for newborn screening, toxicology, and therapeutic monitoring.
Pilot or Case Example: A 2025 hospital pilot showed a 25% reduction in diagnostic turnaround time after implementing integrated mass spectrometry workflows.
Competitive Landscape: Market leader ~39% share (U.S. dominance), major competitors include Thermo Fisher Scientific, Agilent Technologies, Bruker, Shimadzu.
Regulatory & ESG Impact: Tight diagnostic regulations and quality standards accelerate validated MS assay adoption; ESG focus on reducing lab waste via automated workflows.
Investment & Funding Patterns: Recent investments exceed USD 480 million in diagnostics R&D, with growing venture funding for AI‑integrated mass spectrometry tools.
Innovation & Future Outlook: Integration of machine learning for spectral interpretation, next‑generation high‑throughput instruments, and cloud‑based analytics shaping future expansion.
The Clinical Mass Spectrometry Market continues to evolve across key industry sectors. Hospitals and clinical diagnostic laboratories lead consumption due to increasing demand for precise biomarker analysis, therapeutic drug monitoring, and metabolic profiling. Pharmaceutical and biotechnological research sectors are expanding mass spectrometry use in proteomics and drug development workflows, driven by innovations in high‑resolution instruments and automated sample preparation solutions. Regulatory frameworks in developed regions are promoting standardized workflows, while emerging markets emphasize cost‑effective platforms and infrastructure growth. Regional consumption patterns show robust growth in North America and Asia‑Pacific, supported by rising healthcare expenditure and chronic disease prevalence. Future trends point to greater integration of AI, miniaturized devices, and personalized medicine applications enhancing workflow efficiency and diagnostic accuracy.
The Clinical Mass Spectrometry Market plays a strategic role in transforming modern diagnostics and healthcare delivery by enabling high‑precision molecular analysis that surpasses traditional methods. Advanced platforms such as next‑generation LC‑MS/MS deliver up to 45% improvement in analytical specificity compared to conventional immunoassays, making them indispensable in therapeutic drug monitoring, endocrinology, and oncology diagnostics. North America dominates in overall system volume, while Europe leads adoption with over 58% of clinical laboratories deploying mass spectrometry platforms across diagnostic and research workflows. By 2028, integration of AI‑driven spectral interpretation is expected to improve throughput efficiency by approximately 37%, reducing analytical turnaround and enhancing laboratory productivity.
Market participants are also aligning with sustainability and compliance objectives; leading laboratory networks have committed to reducing reagent waste by 22% through automated sample handling by 2030 as part of broader ESG performance targets. In 2025, a major hospital network in Europe achieved a 28% reduction in diagnostic errors after implementing an AI‑enhanced mass spectrometry initiative, demonstrating measurable clinical impact through technology adoption. Strategic investments in modular, automated systems are increasingly prioritized to support decentralized and point‑of‑care testing, particularly in Asia‑Pacific growth corridors.
Looking ahead, the Clinical Mass Spectrometry Market will remain a pillar of resilience, compliance, and sustainable growth as precision diagnostics, digital integration, and workflow automation continue to reframe clinical and research paradigms.
The surge in demand for precision diagnostics is a major driver of the Clinical Mass Spectrometry Market. Clinical laboratories report increased utilization of mass spectrometry platforms for complex assays, including therapeutic drug monitoring, endocrinology analysis, and biomarker quantification. Over 68% of diagnostic labs have integrated clinical mass spectrometry systems into routine workflows to improve diagnostic accuracy and reduce analytical errors, particularly in hormone and metabolic disorder testing. The ability to analyze multiple analytes in a single run with high specificity enhances clinical confidence and supports evidence‑based treatment decisions across specialties such as oncology and toxicology. This shift has bolstered instrument procurement and prompted service expansion in major hospital networks and reference labs. Enhanced automation and digital data processing in mass spectrometry also contribute to higher throughput, enabling laboratories to meet growing sample volumes and complex clinical demands.
High capital investment and operational complexity remain notable restraints for the Clinical Mass Spectrometry Market. Advanced mass spectrometry instruments, including LC‑MS/MS systems, often require significant upfront expenditure, which can be prohibitive for small and mid‑sized laboratories. Beyond initial acquisition, ongoing maintenance, specialized consumables, and regular calibration add to the total cost of ownership. In addition, the complexity of mass spectrometry workflows demands trained technical personnel with expertise in instrument operation, method development, and data interpretation. Many laboratories encounter challenges in recruiting and retaining such specialists, limiting full utilization and slowing adoption rates. Furthermore, integration of complex data outputs into existing laboratory information systems and quality workflows can increase operational burdens, creating barriers for facilities seeking streamlined diagnostic processes.
Expansion into emerging healthcare markets and clinical genomics represents a significant opportunity for the Clinical Mass Spectrometry Market. Rising healthcare expenditure, modernization of laboratory infrastructure, and increased regulatory emphasis on precision diagnostics in regions such as Asia‑Pacific and Latin America have stimulated demand for mass spectrometry solutions. Growth in genomic and proteomic research is driving broader utilization of MS systems for hereditary disease screening, biomarker discovery, and personalized treatment planning. Emerging markets have experienced notable year‑on‑year increases in instrument installations as hospitals and diagnostic chains prioritize high‑performance analytical platforms. The diversification of clinical applications — including metabolomics, vitamin analysis, and toxicology panels — also broadens market potential. Compact and automated system designs further lower barriers to adoption, enabling secondary and regional laboratories to integrate advanced diagnostics previously confined to centralized facilities.
Regulatory complexity and data standardization issues present ongoing challenges for the Clinical Mass Spectrometry Market. Stringent validation requirements and diverse regional approval standards can extend deployment timelines for new instruments and assays, increasing operational costs and administrative workload for manufacturers and end‑users alike. Variability in calibration and quality assurance protocols across laboratories can result in inconsistent analytical outcomes, hindering cross‑facility comparability and confidence in clinical data. Data complexity further strains laboratory IT infrastructure, as high‑volume spectral outputs necessitate robust software ecosystems for processing, storage, and secure integration with broader information systems. These challenges require coordinated efforts to develop harmonized standards, streamlined compliance pathways, and interoperable data management solutions to support scalable mass spectrometry adoption in clinical environments.
Expansion of AI-Driven Analytical Platforms: Artificial intelligence integration in clinical mass spectrometry is transforming laboratory workflows. Over 62% of leading diagnostic labs have implemented AI-enabled spectral analysis, improving sample throughput by 28% and reducing manual interpretation errors by 34%. North America leads adoption, while Asia-Pacific shows rapid uptake with 41% of laboratories deploying AI-assisted platforms for routine testing.
Miniaturization and Portable Instrumentation: Compact, portable mass spectrometry units are increasingly utilized for point-of-care diagnostics and field testing. In 2025, approximately 48% of secondary laboratories adopted benchtop LC-MS/MS systems, achieving a 22% reduction in sample preparation time and expanding clinical testing capabilities in remote and decentralized settings. Europe accounts for 35% of portable instrument installations.
Automation in Sample Preparation and Handling: Automated sample prep systems are reducing labor costs and enhancing consistency in clinical workflows. Over 57% of high-volume hospitals integrated robotic liquid handling for mass spectrometry assays, cutting sample processing time by 31% and minimizing human errors by 27%. The trend is strongest in North America, followed by emerging adoption in Asia-Pacific laboratories.
Growth in Multiplexed Assays and Expanded Clinical Applications: Multiplexed mass spectrometry assays are increasingly applied in newborn screening, toxicology, and metabolomics. By 2025, 44% of clinical laboratories adopted multiplexed LC-MS/MS assays, enabling simultaneous analysis of up to 12 analytes per run, improving diagnostic efficiency and expanding testing portfolios, particularly in hospitals and reference labs across Europe and the U.S.
The Clinical Mass Spectrometry Market is segmented into types, applications, and end-users, offering valuable insight for strategic decision-making. Type segmentation includes diverse mass spectrometry platforms such as LC-MS, GC-MS, tandem MS, ICP-MS, and MALDI, each addressing specific clinical workflows. Applications range from clinical diagnostics, drug development, toxicology screening, proteomics, and metabolomics analysis, reflecting broad industry adoption. End-users include hospitals, diagnostic laboratories, pharmaceutical and biotechnology companies, academic and research institutions, and contract research organizations. North America and Europe show high adoption for hospital and research-based applications, while Asia-Pacific demonstrates rapid uptake in pharmaceutical and clinical testing laboratories. Understanding these segments helps stakeholders identify investment priorities, optimize deployment strategies, and tailor product development to meet the evolving clinical and research demands across regions.
Liquid Chromatography Mass Spectrometry (LC-MS) is the leading product type, accounting for approximately 38% of adoption due to its versatility in analyzing proteins, metabolites, and small molecules with high sensitivity and specificity. Tandem Mass Spectrometry (MS/MS) is the fastest-growing type, driven by its use in newborn screening and therapeutic drug monitoring, with adoption expected to surpass 28% by 2033. Gas Chromatography Mass Spectrometry (GC-MS), Inductively Coupled Plasma Mass Spectrometry (ICP-MS), and Matrix-Assisted Laser Desorption/Ionization (MALDI) collectively represent 34% of the market, serving niche applications such as environmental testing, elemental analysis, and proteomics research.
Clinical diagnostics is the leading application, holding 41% of market adoption due to widespread use in hospitals and reference laboratories for hormone analysis, toxicology, and biomarker detection. The fastest-growing application is drug development and pharmacokinetics, with adoption rising rapidly to support high-throughput analysis in pharmaceutical R&D and personalized medicine initiatives. Toxicology screening, proteomics, metabolomics, and food/environmental testing collectively account for 36% of applications, fulfilling specialized clinical and research needs.
Hospitals and diagnostic laboratories are the dominant end-users, representing 44% of market adoption due to high-volume clinical testing and integration of advanced mass spectrometry platforms for routine diagnostics. The fastest-growing end-user segment is pharmaceutical and biotechnology companies, driven by increasing use of MS for proteomics, drug discovery, and metabolomics research, expected to surpass 27% adoption by 2033. Academic research centers, contract research organizations, and specialized clinical laboratories together account for 29% of the market.
North America accounted for the largest market share at 39% in 2025 however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 12.8% between 2026 and 2033.
North America maintains a strong installed base of clinical mass spectrometry systems, with more than 8,500 operational units across hospitals and reference laboratories. Europe follows with 28% share, supported by over 3,200 clinical laboratories performing routine LC-MS/MS assays. Asia-Pacific represents 22% of global demand, with China, Japan, and India together contributing nearly 61% of regional installations. South America and the Middle East & Africa collectively account for 11%, reflecting expanding healthcare infrastructure and diagnostic modernization programs. Across regions, over 67% of high-complexity laboratories now utilize tandem mass spectrometry for toxicology and hormone testing, while automation adoption has surpassed 52% in large hospital networks, indicating a global shift toward high-throughput, digitally integrated diagnostic platforms.
How are advanced diagnostics and regulatory frameworks accelerating high-precision laboratory adoption?
This region holds approximately 39% of global clinical mass spectrometry installations, driven by strong demand from hospitals, reference laboratories, and pharmaceutical research facilities. Key industries include clinical diagnostics, drug development, and toxicology testing, where more than 72% of large laboratories employ LC-MS/MS platforms. Regulatory oversight emphasizing laboratory quality standards and validated diagnostic workflows supports sustained system upgrades. Digital transformation is evident, with over 58% of laboratories integrating AI-based data analysis tools into mass spectrometry operations. A leading U.S.-based analytical instrument provider expanded automated sample preparation solutions in 2025, increasing workflow efficiency by 30% across partner hospitals. Consumer behavior reflects higher adoption of advanced diagnostic testing, particularly in specialized healthcare and precision medicine programs, where demand for multi-analyte testing panels continues to grow.
What role do quality standards and research intensity play in laboratory technology adoption?
Europe represents 28% of global clinical mass spectrometry demand, with Germany, the UK, and France collectively accounting for nearly 55% of regional system usage. Regulatory frameworks emphasize analytical accuracy and standardized laboratory procedures, encouraging deployment of high-resolution MS platforms. Sustainability initiatives have led 46% of large laboratories to adopt automated systems that reduce solvent consumption and laboratory waste. Emerging technologies such as multiplexed assays and digital data platforms are increasingly implemented, with 49% of university hospitals using integrated informatics with mass spectrometry workflows. A German analytical instrumentation firm enhanced high-throughput screening solutions in 2025, enabling clinical labs to process 26% more samples daily. Regional consumer behavior shows a preference for validated, regulation-compliant diagnostic technologies that ensure data transparency and reproducibility.
How is healthcare modernization influencing laboratory technology expansion?
Asia-Pacific ranks third in overall market volume but leads in installation growth momentum. China, Japan, and India together represent over 61% of regional consumption, supported by more than 2,400 newly established diagnostic laboratories since 2022. Infrastructure expansion includes hospital modernization projects and research facility development, with 54% of tertiary hospitals adopting advanced LC-MS systems. Innovation hubs in Japan and South Korea focus on miniaturized and portable MS technologies for decentralized diagnostics. A Japanese instrument manufacturer introduced compact MS systems in 2025, reducing instrument footprint by 35% while maintaining analytical performance. Regional consumer patterns indicate growing demand for affordable, automated diagnostic solutions as healthcare access expands in urban and semi-urban centers.
How are healthcare investments and modernization programs shaping diagnostic capabilities?
Brazil and Argentina are the primary markets, contributing nearly 63% of regional demand. South America holds around 6% of global clinical mass spectrometry installations, with diagnostic laboratory modernization programs increasing system adoption in metropolitan hospitals. Infrastructure improvements include laboratory automation upgrades, with 38% of large urban labs deploying automated sample handling systems. Government healthcare investments emphasize improved diagnostic accuracy and disease monitoring. A Brazilian laboratory equipment distributor expanded partnerships with clinical centers in 2025, improving assay processing capacity by 24%. Regional consumer trends show rising reliance on centralized diagnostic laboratories, where demand for high-precision hormone and toxicology testing is increasing steadily.
How is laboratory modernization supporting advanced diagnostic adoption?
This region represents approximately 5% of global installations, with the UAE and South Africa leading demand. Healthcare modernization initiatives have resulted in 41% of tertiary hospitals adopting advanced diagnostic platforms. Technological progress includes digital laboratory systems and automated workflows, now used by 36% of major clinical labs. Trade partnerships and healthcare infrastructure investments support imports of advanced analytical instruments. A regional healthcare group in the UAE implemented automated LC-MS workflows in 2025, improving sample throughput by 29%. Consumer behavior indicates growing preference for specialized diagnostic testing in urban centers, while public health initiatives promote expanded access to metabolic and toxicology screening services.
United States – 34% share: Strong clinical research infrastructure and high adoption of advanced diagnostic platforms support leadership in the Clinical Mass Spectrometry Market.
Germany – 11% share: Robust laboratory quality standards and extensive biomedical research activities drive consistent demand in the Clinical Mass Spectrometry Market.
The Clinical Mass Spectrometry market exhibits a moderately consolidated structure, with the top 5 companies collectively accounting for approximately 58% of global system installations. More than 45 active manufacturers and technology providers compete across instrument platforms, consumables, and software solutions. Market leaders focus on high-resolution LC-MS/MS systems, automated sample preparation, and integrated data analysis platforms, which together represent over 63% of new laboratory procurements. Strategic initiatives have intensified, with over 18 product launches recorded in the past two years emphasizing compact designs, AI-enabled analytics, and workflow automation. Partnerships between instrument manufacturers and clinical laboratory networks increased by 27% since 2024, aimed at co-developing validated diagnostic assays. Mergers and acquisitions remain active, with at least 9 technology-driven acquisitions strengthening software and digital integration capabilities. Innovation trends center on miniaturization, multiplexing capacity exceeding 10 analytes per run, and cloud-based data systems adopted by 46% of high-complexity labs. Competitive positioning increasingly depends on service networks, regulatory compliance support, and end-to-end automation offerings that reduce manual intervention by up to 30%.
Thermo Fisher Scientific
Agilent Technologies
Bruker Corporation
Shimadzu Corporation
Waters Corporation
SCIEX
PerkinElmer
JEOL Ltd.
LECO Corporation
Analytik Jena
Technological advancement remains central to the evolution of the Clinical Mass Spectrometry Market, with innovation focusing on sensitivity, automation, and digital integration. High-resolution LC-MS/MS systems now achieve detection limits below 1 ng/mL for multiple analytes, supporting highly accurate hormone profiling, toxicology screening, and metabolic disorder testing. More than 64% of high-complexity clinical laboratories have transitioned from single-analyte immunoassays to multiplexed mass spectrometry workflows capable of analyzing 8–15 compounds in a single run, increasing testing efficiency by over 30%.
Automation technologies are reshaping laboratory operations, with robotic liquid handling and automated sample preparation platforms deployed in 57% of large hospital laboratories. These systems reduce manual intervention by approximately 28% and lower pre-analytical error rates by 22%. Integration with laboratory information systems and cloud-based data platforms has expanded, with 48% of advanced laboratories using digital data management solutions for real-time quality control and secure data sharing.
Artificial intelligence and machine learning algorithms are gaining prominence in spectral interpretation, enabling up to 35% faster data processing and improved anomaly detection. Miniaturized and benchtop MS instruments, now accounting for nearly 31% of new installations, support decentralized testing environments and smaller laboratories. Additionally, ion mobility spectrometry coupled with mass spectrometry enhances molecular separation, improving compound identification accuracy by nearly 20% in complex biological samples. These technological trends collectively enhance precision, scalability, and operational efficiency across clinical diagnostic settings.
• In June 2025, Thermo Fisher Scientific launched the Orbitrap Astral Zoom mass spectrometer, offering approximately 30% faster scan speeds, 40% higher throughput, and 50% expanded multiplexing capabilities designed to support large-scale proteomics and biomarker discovery initiatives.
• In June 2025, Agilent Technologies introduced the InfinityLab™ Pro iQ Series LC/MS instruments featuring up to 25% sensitivity gains and intelligent mass detection for enhanced analytical performance across clinical laboratories, particularly in small and large molecule analysis.
• In 2025, Bruker Corporation rolled out the timsMetabo™ mass spectrometer with advanced 4D separations, delivering 10x improvement in small molecule analyses for metabolomics and lipidomics, significantly boosting annotation confidence and automated identification.
• In 2024, Thermo Fisher Scientific showcased the Stellar mass spectrometer, a high-throughput, high-sensitivity platform designed to integrate with UHPLC workflows and improve translational omics research efficiency in clinical and biopharma laboratories.
The Clinical Mass Spectrometry Market Report offers a comprehensive view of industry developments across key segments, technologies, and regions. Included are detailed evaluations of instrument types such as LC-MS, GC-MS, tandem MS systems, ICP-MS, and MALDI, with insights into performance capabilities, sensitivity levels, and application fit across clinical workflows. The report maps application areas including clinical diagnostics, therapeutic drug monitoring, toxicology screening, proteomics, metabolomics, and niche uses such as environmental and food testing.
Geographic analysis assesses market behavior in North America, Europe, Asia-Pacific, South America, and the Middle East & Africa, highlighting adoption patterns, infrastructure development, and regulatory influences on deployment strategies. Technology focus areas cover automation in sample preparation, AI and machine learning integration for spectral data interpretation, digital connectivity for laboratory information management, and trends in compact and portable MS solutions.
Insight into end-user segments — including hospitals, diagnostic laboratories, pharmaceutical and biotech firms, research institutions, and contract research organizations — provides clarity on adoption drivers, operational demands, and consumer behavior variations. Emerging niches such as decentralized diagnostics, point-of-care mass spectrometry, and multi-omics platforms are examined for long-term strategic relevance. The report also explores innovation ecosystems, partnership models, and competitive landscapes that influence product development and commercial strategies within the clinical mass spectrometry domain.
| Report Attribute/Metric | Report Details |
|---|---|
|
Market Revenue in 2025 |
USD V2025 Million |
|
Market Revenue in 2033 |
USD V2033 Million |
|
CAGR (2026 - 2033) |
10.5% |
|
Base Year |
2025 |
|
Forecast Period |
2026 - 2033 |
|
Historic Period |
2021 - 2025 |
|
Segments Covered |
By Types
By Application
By End-User
|
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Region Covered |
North America, Europe, Asia-Pacific, South America, Middle East, Africa |
|
Key Players Analyzed |
Thermo Fisher Scientific , Agilent Technologies , Bruker Corporation , Shimadzu Corporation, Waters Corporation, SCIEX, PerkinElmer, JEOL Ltd., LECO Corporation, Analytik Jena |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
