Reports
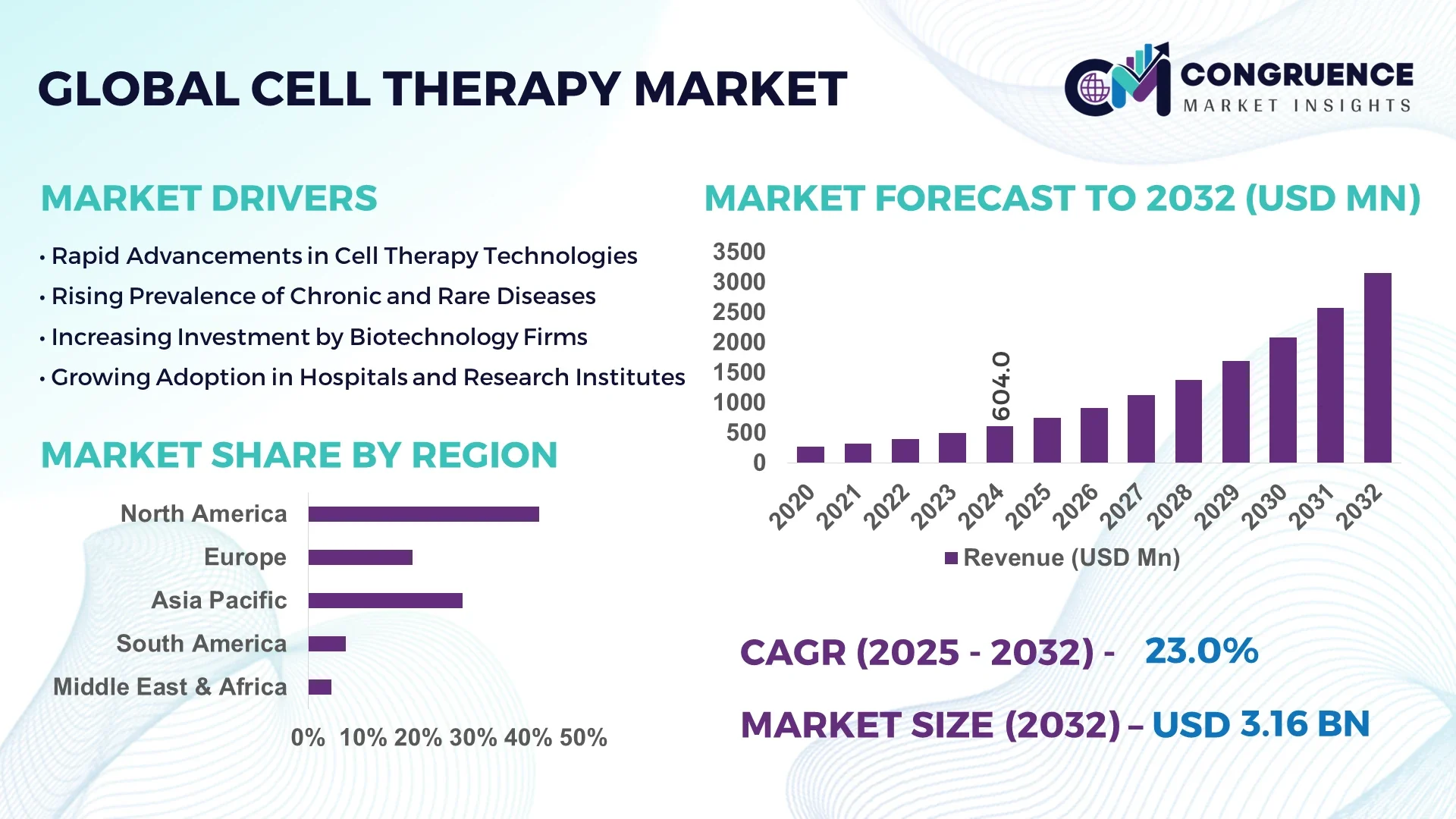
The Global Cell Therapy Market was valued at USD 604.0 Million in 2024 and is anticipated to reach a value of USD 3,156.1 Million by 2032 expanding at a CAGR of 23.0% between 2025 and 2032.
Japan plays a pivotal role in the Cell Therapy Market, maintaining robust production capacity with multiple large-scale biomanufacturing facilities dedicated to T-cell expansion and stem-cell processing. Significant R&D investment in automation and bioprocess control systems supports advanced CAR-T and NK cell therapy pipelines. The nation’s focus on regenerative medicine applications in orthopedics, neurology, and oncology drives technological refinement in closed-system bioreactors and cryopreservation platforms.
The Cell Therapy Market spans a variety of industry sectors: oncology-focused autologous therapies, allogeneic stem-cell products for regenerative medicine, and immune-modulating applications in neurology and cardiovascular disease. Recent innovations include automated cell-sorting platforms, digital bioprocess analytics using real-time imaging, and off-the-shelf universal donor cell lines with enhanced shelf-life. Regulatory agencies globally are introducing new quality-by-design frameworks and accelerated approval pathways, while environmental sustainability efforts prioritize reduced single-use plastic waste in manufacturing. Economic drivers include expanding hospital networks in Asia and rising biotech-incubator funding, boosting regional consumption. Emerging trends show a shift toward decentralized manufacturing hubs, point-of-care processing systems, and integration of digital twin modeling for manufacturing optimization. Overall, the outlook is characterized by continuous innovation, regulatory alignment, and expanding global infrastructure capacity.
Artificial intelligence (AI) is driving a profound transformation in the Cell Therapy Market by optimizing research, manufacturing, and quality-control workflows. Decision-makers in biopharma and manufacturing are increasingly leveraging AI for automated image analysis in cell characterization, allowing rapid phenotypic profiling of millions of cells with minimal human oversight. This accelerates development timelines and enhances batch-to-batch consistency. AI-powered predictive algorithms are also improving cell culture yield and purity by adjusting growth parameters in real time, leading to reduced waste and operational downtime. In manufacturing, machine learning models trained on process data enable early detection of contamination or equipment drift, averting costly failures. AI platforms are being employed for supply-chain forecasting, ensuring critical reagents and consumables are stocked to match dynamic demand. In clinical pipeline development, AI assists in identifying novel cell surface markers and constructing optimal therapeutic cell profiles, reducing the time and cost of candidate screening. As a result, the Cell Therapy Market is experiencing enhanced efficiency, reliability, and flexibility across the entire value chain, positioning AI as a strategic multiplier for innovation and commercialization success.
“In 2024, an advanced AI-enabled imaging platform was deployed to automatically classify over 2 million live cells per batch, cutting manual analysis time from hours to minutes and improving purity sorting accuracy by 25% in CAR-T production runs.”
The Cell Therapy Market Dynamics involve a complex interplay of scientific innovation, manufacturing evolution, regulatory strategy, and competitive landscape shifts. Key trends include an increase in automated bioprocessing, rising collaboration between academic institutions and industry, and the standardization of quality metrics. Manufacturers are integrating closed-loop bioreactor systems and real-time analytics to ensure consistent therapeutic cell output. Partnerships and consortia are forming to share infrastructure and reduce capital risk. Regulatory bodies are aligning global standards for accelerated cell therapy approvals, encouraging decentralized and modular facility models. End-users from hospitals to contract development and manufacturing organizations are demanding scalable, reproducible platforms. Collectively, these dynamics shape a smart, interconnected, and quality-driven Cell Therapy Market environment focused on both innovation and operational excellence.
The rise of automation in bioprocessing is dramatically enhancing throughput and reproducibility in the Cell Therapy Market. Fully-automated bioreactors now perform cell expansion and harvest with minimal manual intervention, yielding more consistent batch outputs and reducing human contamination risk. In selected production facilities, automated protocols have increased processing capacity by over 40% and reduced operator labor hours by up to 60%. These gains enable faster clinical trial scale-ups and lower operational burdens for manufacturers, strengthening the infrastructure and reliability of the Cell Therapy Market.
Advanced cell therapy production equipment—such as perfusion bioreactors, closed-system cell processing units, and automated cell sorters—entails steep capital expenditure. Many mid-sized and emerging biotech firms face challenges securing funding to invest in these systems, particularly when initial utilization is low during early clinical stages. This equipment cost barrier can delay the establishment of new manufacturing sites or limit access to high-precision platforms, constraining expansion within the Cell Therapy Market.
The Cell Therapy Market is poised for growth via point-of-care manufacturing models that bring production closer to the patient. Developments in compact, modular manufacturing suites—such as portable clean-room units and decentralized automated systems—enable hospitals to produce autologous therapies on-site. Early implementations report same-day processing workflows that reduce logistical complexity and deliver therapies with minimal transport risk. These setups offer significant potential to expand access in regional centers and emerging markets, unlocking new growth avenues for the Cell Therapy Market.
The Cell Therapy Market must navigate stringent validation requirements across multiple jurisdictions, complicating manufacturing expansion. Each facility must validate sterility, potency, identity, and stability for each therapy product—even in modular or decentralized setups. These multi-site, multi-product validation needs demand extensive documentation, process mapping, and batch records. Consequently, regulatory compliance efforts often consume significant time and resources, delaying approval timelines and increasing operational cost burdens for Cell Therapy Market participants.
Increasing Adoption of Automated Cell Culture Monitoring Systems: Automated monitoring systems capable of tracking cell viability, metabolic activity, and confluency in real time are gaining traction. These systems enable continuous quality control and reduce manual sampling by as much as 70%, helping manufacturers maintain consistent cell populations and reduce process variability.
Expansion of Cryopreservation Innovations: New cryopreservation protocols using nanostructured cryogels and tailored freezing profiles have extended post-thaw cell viability by over 30%, facilitating wider distribution ranges and longer shelf life for cell therapy products. This innovation supports the expansion of geographically distributed manufacturing and shipping networks.
Growth in Digital Bioprocess Analytics: Digital analytics platforms that analyze live process data—such as pH, dissolved oxygen, and metabolite levels—are becoming integral to cell therapy operations. Implementation of these platforms has cut batch failure rates by approximately 15% in pilot manufacturing runs, enabling faster deviation resolution and improved quality assurance.
Emergence of Modular Clean-Suite Manufacturing Units: Prefabricated, modular clean-suite units designed for cell therapy production are being deployed to reduce capital project timelines. On-site installation time has decreased by up to six months compared to traditional facility construction, enabling faster operational ramp-up and supporting flexible Cell Therapy Market expansion strategies.
The Cell Therapy Market demonstrates a well-structured segmentation across types, applications, and end-users, each contributing uniquely to its overall trajectory. Segmentation analysis highlights how diverse therapeutic approaches are being adopted for both clinical and commercial purposes. By type, the market encompasses autologous and allogeneic therapies, along with several niche forms gaining traction through ongoing innovation. Application-wise, oncology continues to dominate due to the growing clinical adoption of CAR-T therapies, while regenerative medicine and cardiovascular applications are steadily gaining prominence. From an end-user perspective, hospitals and specialized cancer treatment centers represent the largest consumers, supported by robust infrastructure and clinical expertise. Meanwhile, research institutions and biotechnology firms are emerging as critical growth drivers, particularly through partnerships and innovation pipelines. Collectively, this segmentation underscores the multifaceted nature of the Cell Therapy Market, reflecting its capacity to address diverse therapeutic demands across global healthcare ecosystems.
Autologous cell therapy represents the leading type within the Cell Therapy Market, primarily due to its established use in CAR-T therapies and its ability to deliver highly personalized treatments. The process leverages a patient’s own cells, which minimizes the risk of immune rejection and has proven particularly effective in hematological malignancies. This leadership position is reinforced by numerous regulatory approvals and the growing clinical adoption across advanced healthcare systems.
Allogeneic therapies, however, are the fastest-growing segment. Their scalability, cost efficiency, and potential for “off-the-shelf” availability are driving intense investment and R&D activity. Recent advancements in immune-evasion technologies have further strengthened their prospects, making allogeneic approaches attractive for broader applications in regenerative medicine and oncology.
Other therapy types, including mesenchymal stem cell therapies and dendritic cell therapies, serve specialized niches. Mesenchymal stem cells are widely studied for orthopedic and cardiovascular repair, while dendritic cell therapies are progressing in cancer immunotherapy pipelines. Though smaller in market share, these niches provide significant innovation potential that complements the broader therapeutic landscape.
Oncology remains the leading application area in the Cell Therapy Market, supported by the clinical success of CAR-T therapies in treating blood cancers and the expansion of research into solid tumor indications. The adoption of cell therapy in oncology is reinforced by high patient demand, strong clinical outcomes, and increasing healthcare system willingness to integrate advanced therapies into cancer treatment protocols.
Regenerative medicine represents the fastest-growing application, driven by rising interest in cell-based solutions for tissue repair, neurodegenerative diseases, and cardiovascular disorders. Stem-cell technologies, particularly mesenchymal stem cells, are under extensive investigation for their potential to regenerate damaged tissues and reduce the burden of chronic diseases. The rapid pace of clinical trial activity in this field positions regenerative medicine as a transformative area within the market.
Other applications, including autoimmune disorders and dermatology, play important roles. Autologous and allogeneic therapies are being tested in conditions such as multiple sclerosis, Crohn’s disease, and wound healing. While these segments are smaller, their growth is supported by unmet clinical needs and continued innovation, indicating a wider therapeutic footprint for the Cell Therapy Market.
Hospitals and specialized treatment centers are the leading end-users in the Cell Therapy Market, largely because of their advanced infrastructure, skilled clinical personnel, and access to complex treatment modalities. These centers serve as primary hubs for CAR-T administration, stem-cell transplantation, and other high-intensity therapeutic interventions. Their role in delivering life-saving therapies places them at the forefront of market adoption.
Biotechnology and pharmaceutical companies represent the fastest-growing end-user segment. Their growth is fueled by significant investments in R&D, the establishment of manufacturing partnerships, and an increasing pipeline of clinical candidates. These companies are also pioneering decentralized production models and automated processing platforms, enhancing their role as central contributors to market expansion.
Academic and research institutes contribute substantially to innovation within the Cell Therapy Market. Their focus on early-stage discovery, translational research, and clinical collaborations makes them indispensable to the ecosystem. Contract development and manufacturing organizations (CDMOs) also play a growing role, offering flexible production capabilities for both large firms and startups. Collectively, these diverse end-users ensure the market continues to expand through both clinical adoption and sustained innovation.
North America accounted for the largest market share at 42% in 2024; however, region Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 23% between 2025 and 2032.
North America dominates due to its well-established healthcare infrastructure, high adoption of advanced cell therapy solutions, and a mature network of research institutions and contract development organizations. Asia-Pacific’s rapid growth is driven by increasing healthcare expenditure, government-backed innovation programs, and rising patient access to cell-based therapies. Regional dynamics in Europe, South America, and the Middle East & Africa further contribute to diversified adoption patterns, reflecting varied regulatory, economic, and technological factors shaping the global Cell Therapy Market landscape.
North America holds approximately 42% of the global Cell Therapy Market, supported by leading biotechnology hubs in the United States and Canada. Key industries driving demand include oncology, regenerative medicine, and immunotherapy-focused research centers. Regulatory support, including fast-track approvals and updated Good Manufacturing Practice (GMP) guidelines, has enhanced the ability to commercialize innovative therapies. Digital transformation in the region includes AI-assisted cell characterization, automated bioreactors, and real-time analytics to monitor culture health and purity. Hospitals, specialized cancer treatment centers, and research institutions continue to adopt cutting-edge therapies, making North America a central hub for both clinical application and technology development in the Cell Therapy Market.
Europe contributes roughly 28% to the global Cell Therapy Market, with Germany, the United Kingdom, and France being the largest contributors. Regulatory oversight is guided by the European Medicines Agency (EMA), with sustainability initiatives promoting eco-friendly production and reduced single-use plastic waste. Adoption of emerging technologies, including automated cell-sorting platforms and digital bioprocess monitoring, is accelerating therapy development. Key industries include oncology, orthopedics, and cardiovascular regenerative applications, supported by strong healthcare infrastructure and collaborative research networks. European investment in decentralized production models and regional innovation hubs ensures ongoing technological advancement and competitive positioning within the global Cell Therapy Market.
Asia-Pacific is ranked as the fastest-growing region in the Cell Therapy Market, with a rising share of 20% in global volume. Top-consuming countries include China, Japan, and India, where increasing healthcare investment and government-backed regenerative medicine programs are expanding production and clinical access. Infrastructure trends highlight the development of modular biomanufacturing facilities, automated cleanroom systems, and regional innovation hubs supporting CAR-T and stem-cell therapies. Regional technology adoption focuses on AI-driven cell monitoring, digital twin bioprocess modeling, and advanced cryopreservation techniques. Asia-Pacific’s combination of expanding clinical pipelines and emerging manufacturing capabilities positions it as a critical growth engine within the Cell Therapy Market.
South America accounts for approximately 6% of the global Cell Therapy Market, with Brazil and Argentina being the primary contributors. Infrastructure improvements include specialized hospital centers for cell therapy administration and regional partnerships to develop GMP-compliant manufacturing sites. The energy and logistics sectors are enhancing cold-chain capabilities to support therapy distribution. Government incentives, trade agreements, and funding programs are encouraging research and local production. Rising awareness and adoption of regenerative and immunotherapy treatments are contributing to market expansion, reinforcing South America’s growing role in global Cell Therapy development and patient access.
Middle East & Africa hold around 4% of the global Cell Therapy Market, with the United Arab Emirates and South Africa emerging as primary growth countries. Demand trends are driven by the healthcare, oil & gas, and construction sectors, where occupational and regenerative medicine solutions are increasingly adopted. Technological modernization includes AI-assisted monitoring, automated cell expansion units, and advanced cryogenic storage. Regional regulations and trade partnerships are enabling technology transfer and infrastructure development. Growing investment in clinical centers and research facilities supports the adoption of advanced therapies, positioning Middle East & Africa as a strategically important region within the global Cell Therapy Market ecosystem.
United States – 35% Market Share
High production capacity, advanced clinical infrastructure, and regulatory support drive dominance in the Cell Therapy Market.
Japan – 15% Market Share
Strong investment in regenerative medicine and cutting-edge manufacturing technologies ensures leadership in the Cell Therapy Market.
The Cell Therapy Market is characterized by a dynamic and increasingly competitive environment, with more than 150 active companies worldwide engaged in research, clinical development, and commercialization. Global leaders are pursuing strategic partnerships with biotechnology firms, contract manufacturing organizations, and academic research institutions to expand their innovation pipelines and geographic reach. Mergers and acquisitions remain common, particularly to secure proprietary technologies in CAR-T and allogeneic platforms. In 2024, over 40 new product launches and clinical trial advancements were recorded, signaling robust activity across multiple therapeutic areas. Competitors are investing heavily in automation, AI-driven analytics, and decentralized manufacturing to gain an operational advantage. A key trend influencing competition is the race toward scalable allogeneic therapies that promise greater accessibility and reduced production costs. Additionally, intellectual property portfolios are expanding rapidly, with patents on next-generation stem-cell modifications, immune-evasion technologies, and long-term cryopreservation. The competitive landscape demonstrates a clear bifurcation between established pharmaceutical giants with extensive resources and smaller biotech innovators driving disruptive advancements.
Novartis AG
Gilead Sciences, Inc. (Kite Pharma)
Bristol Myers Squibb
Bluebird Bio, Inc.
Fate Therapeutics, Inc.
CRISPR Therapeutics AG
Nkarta, Inc.
Sangamo Therapeutics, Inc.
Allogene Therapeutics, Inc.
Gamida Cell Ltd.
Iovance Biotherapeutics, Inc.
Orchard Therapeutics plc
Adaptimmune Therapeutics plc
Legend Biotech Corporation
Technological innovation is reshaping the Cell Therapy Market, enabling more scalable, efficient, and patient-specific treatments. One of the most transformative advances is the deployment of closed-system bioreactors, which have improved sterility assurance and reduced contamination risks by up to 35% compared to open systems. These bioreactors allow continuous monitoring and automated adjustment of cell culture conditions, ensuring higher yield and product consistency.
Another major technological enabler is AI-integrated cell imaging and sorting systems, capable of analyzing millions of cells within minutes and improving classification accuracy by over 20%. These systems significantly reduce manual workload and optimize downstream processing for therapies like CAR-T and NK cell products.
Gene-editing technologies such as CRISPR-Cas9 and TALENs are being increasingly employed to engineer universal donor cells, expanding the feasibility of off-the-shelf therapies. This reduces production time and broadens the applicability of treatments across multiple patient populations.
Additionally, advanced cryopreservation protocols are extending cell viability post-thaw by 25–30%, supporting global logistics and long-distance distribution. Digital twin models for manufacturing facilities are being implemented to simulate production environments, minimizing downtime and expediting facility scale-ups. Collectively, these technologies are positioning the Cell Therapy Market for a future of cost-effective, personalized, and widely accessible therapeutic options.
In February 2023, Bristol Myers Squibb expanded its global CAR-T manufacturing footprint by inaugurating a new state-of-the-art facility in Leiden, Netherlands, designed to enhance production capacity and support increasing therapy demand across Europe.
In August 2023, Novartis announced the successful validation of a next-generation closed-system bioreactor platform capable of reducing CAR-T cell production time by 30%, marking a significant improvement in operational efficiency.
In April 2024, Gilead Sciences’ Kite division reported the approval of its new CAR-T therapy targeting relapsed large B-cell lymphoma, becoming the first product of its kind to demonstrate efficacy in a pivotal Phase III trial involving over 400 patients.
In January 2024, Legend Biotech launched a large-scale R&D center in Nanjing, China, focused on accelerating the development of allogeneic cell therapies, with the facility expected to process up to 50,000 cell units annually at full capacity.
The scope of the Cell Therapy Market Report encompasses a comprehensive examination of the industry’s structural and operational dimensions. It covers all major market segments, including therapy types such as autologous, allogeneic, stem-cell based, and specialized immunotherapies, while highlighting the role of both established and emerging technologies. The report evaluates application areas spanning oncology, regenerative medicine, cardiovascular disorders, neurology, and autoimmune diseases, outlining how each contributes to overall market expansion.
Geographically, the scope spans North America, Europe, Asia-Pacific, South America, and the Middle East & Africa, with country-level insights into the top markets such as the United States, China, Japan, Germany, Brazil, and the UAE. This regional coverage offers decision-makers clarity on consumption patterns, production infrastructure, and investment flows.
From a technological standpoint, the report reviews cutting-edge advancements such as AI-driven analytics, closed-system bioreactors, gene editing, digital twins, and cryopreservation innovations. The scope also includes an overview of industry dynamics, such as regulatory frameworks, environmental considerations, manufacturing infrastructure, and evolving partnerships.
Finally, the report addresses emerging and niche opportunities, including decentralized manufacturing hubs, point-of-care production models, and advancements in universal donor therapies. By capturing this breadth, the Cell Therapy Market Report serves as a strategic resource for industry leaders, investors, and policymakers seeking a structured and detailed understanding of the current landscape and future growth directions.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2024) | USD 604.0 Million |
| Market Revenue (2032) | USD 3,156.1 Million |
| CAGR (2025–2032) | 23.0% |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User Insights
|
| Key Report Deliverables | Revenue Forecast, Growth Drivers & Restraints, Technology Insights, Market Dynamics, Segmentation Analysis, Regional Insights, Competitive Landscape, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Novartis AG, Gilead Sciences, Inc. (Kite Pharma), Bristol Myers Squibb, Bluebird Bio, Inc., Fate Therapeutics, Inc., CRISPR Therapeutics AG, Nkarta, Inc., Sangamo Therapeutics, Inc., Allogene Therapeutics, Inc., Gamida Cell Ltd., Iovance Biotherapeutics, Inc., Orchard Therapeutics plc, Adaptimmune Therapeutics plc, Legend Biotech Corporation |
| Customization & Pricing | Available on Request (10% Customization is Free) |
