Reports
The Global Cardiac Biomarkers Testing Market is expected to expand at a CAGR of 11.7% between 2023 and 2030. Cardiac biomarkers testing is pivotal in the diagnosis and management of cardiovascular diseases, facilitating the identification of specific proteins released into the bloodstream during instances of cardiac injury or stress. Troponin, CK-MB, BNP, and NT-proBNP are among the commonly assessed biomarkers, analyzed through either blood tests or point-of-care (POC) tests. Technological advancements, particularly in immunoassays and high-sensitivity assays, have markedly enhanced the precision and efficacy of biomarker detection methods. Given the escalating global prevalence of cardiovascular diseases, there exists a growing imperative for cardiac biomarkers testing, thereby propelling market expansion. Key industry players are providing a comprehensive array of testing solutions to healthcare practitioners worldwide.
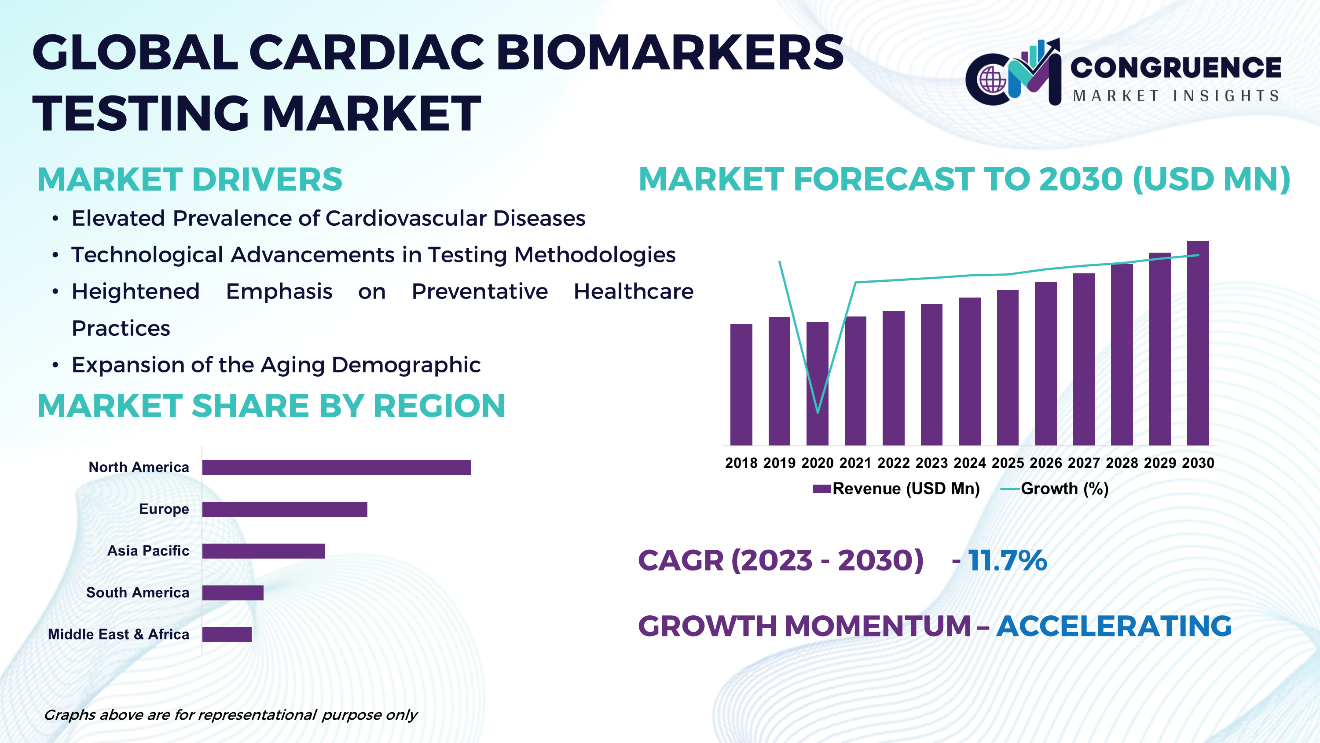
Cardiac Biomarkers Testing Market Major Driving Forces
Elevated Prevalence of Cardiovascular Diseases: The escalating global incidence of cardiovascular ailments, encompassing heart attacks, strokes, and heart failure, underscores the imperative for cardiac biomarkers testing. Given the persistent prominence of these conditions as primary sources of morbidity and mortality, there exists an augmented necessity for precise and expeditious diagnostic procedures to inform treatment modalities and care protocols.
Technological Advancements in Testing Methodologies: Ongoing strides in diagnostic technologies, inclusive of immunoassays, high-sensitivity assays, and point-of-care testing, foster enhanced sensitivity, specificity, and efficiency in cardiac biomarkers testing. These innovative developments facilitate expedited and more accurate identification of cardiac biomarkers, thereby refining patient outcomes and facilitating timely intervention.
Heightened Emphasis on Preventative Healthcare Practices: With an amplified awareness regarding the significance of preventive healthcare measures, there emerges a discernible trend towards proactive screening and early identification of cardiovascular risk factors. In this context, cardiac biomarkers testing assumes a pivotal role in risk stratification, empowering healthcare professionals to discern individuals predisposed to cardiovascular ailments and implement requisite preventive measures accordingly.
Expansion of the Aging Demographic: The expanding elderly demographic on a global scale contributes substantively to the escalating incidence of cardiovascular maladies, given the inherent association between advancing age and heightened cardiovascular risk. Consequently, as the geriatric population burgeons, the demand for cardiac biomarkers testing is poised to intensify, driven by the necessity for comprehensive cardiovascular risk assessment and ailment management among older cohorts.
Cardiac Biomarkers Testing Market Key Opportunities
Remote Monitoring and Telemedicine: The proliferation of telemedicine and remote monitoring technologies creates opportunities for remote cardiac biomarkers testing and monitoring. Home-based testing kits and wearable devices equipped with biomarker sensors enable real-time monitoring of cardiac health, empowering patients and healthcare providers with valuable insights for proactive management.
Point-of-Care Testing: The increasing demand for rapid and convenient diagnostic solutions drives opportunities for point-of-care testing in cardiac biomarkers. Portable and easy-to-use testing devices enable faster decision-making in emergency departments, ambulances, and primary care settings, improving patient outcomes through timely intervention.
Personalized Medicine: Advancements in precision medicine and molecular diagnostics pave the way for personalized approaches to cardiovascular care. Tailoring treatment strategies based on individual patient profiles, including genetic predispositions and biomarker levels, can optimize therapeutic efficacy and minimize adverse effects.
Integration of Artificial Intelligence (AI) and Big Data: Leveraging AI algorithms and big data analytics enables the interpretation of complex datasets generated from cardiac biomarkers testing. Predictive analytics models can identify patterns, trends, and predictive markers for cardiovascular risk, facilitating proactive intervention and preventive strategies.
Cardiac Biomarkers Testing Market Key Trends
· Increasing use of assays detecting lower biomarker concentrations for early diagnosis and risk stratification.
· Simultaneous measurement of multiple biomarkers in a single sample for comprehensive cardiovascular assessment.
· Incorporation of cardiac biomarkers testing into decentralized platforms for rapid diagnosis and treatment in various healthcare settings.
· Emphasis on identifying biomarkers predicting cardiovascular disease onset or progression for proactive interventions.
· Tailoring treatment strategies based on individual patient profiles and genetic factors revealed by cardiac biomarkers testing.
· AI-driven analysis of large datasets for predictive analytics, risk stratification, and treatment optimization.
Market Competition Landscape
The market competition landscape in cardiac biomarkers testing is characterized by intense rivalry among leading players striving for market share. Companies engage in product differentiation, innovation, and strategic collaborations to gain a competitive edge. Additionally, stringent regulatory standards and evolving healthcare policies shape market dynamics. Emerging players often face barriers to entry due to established incumbents and high research and development costs.
Key players in the global Cardiac Biomarkers Testing market implement various organic and inorganic strategies to strengthen and improve their market positioning. Prominent players in the market include:
· Roche Diagnostics Corporation
· Abbott Laboratories
· Siemens Healthcare GmbH
· Beckman Coulter, Inc. (Danaher Corporation)
· bioMérieux
· Ortho Clinical Diagnostics (Carlyle Group)
· Bio-Rad Laboratories, Inc.
· Becton, Dickinson and Company
· Thermo Fisher Scientific Inc.
· Fujirebio (Miraca Holdings Inc.)
· Tosoh Corporation
· Quidel Corporation
· Singulex, Inc.
· Abcam plc
· Bio-Techne Corporation
· Luminex
· DiaSorin S.p.A.
· Trivitron Healthcare
|
Report Attribute/Metric |
Details |
|
Base Year |
2022 |
|
Forecast Period |
2023 – 2030 |
|
Historical Data |
2018 to 2022 |
|
Forecast Unit |
Value (US$ Mn) |
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Segments Covered |
· By Biomarker Type (Troponin, CK-MB (Creatine Kinase-MB), BNP (B-type Natriuretic Peptide) and NT-proBNP, and Others) · By Test Type (Blood Tests, and Point-of-Care (POC) Tests) · By Application (Acute Coronary Syndrome (ACS), Heart Failure, and Other Cardiovascular Conditions) · By End-User (Hospitals, Diagnostic Laboratories, Ambulatory Care Centers, and Others) |
|
Geographies Covered |
North America: U.S., Canada and Mexico Europe: Germany, France, U.K., Italy, Spain, and Rest of Europe Asia Pacific: China, India, Japan, South Korea, Southeast Asia, and Rest of Asia Pacific South America: Brazil, Argentina, and Rest of Latin America Middle East & Africa: GCC Countries, South Africa, and Rest of Middle East & Africa |
|
Key Players Analyzed |
Roche Diagnostics Corporation, Abbott Laboratories, Siemens Healthcare GmbH, Beckman Coulter, Inc. (Danaher Corporation), bioMérieux, Ortho Clinical Diagnostics (Carlyle Group), Bio-Rad Laboratories, Inc., Becton, Dickinson and Company, Thermo Fisher Scientific Inc., Fujirebio (Miraca Holdings Inc.), Tosoh Corporation, Quidel Corporation, Singulex, Inc., Abcam plc, Bio-Techne Corporation, Luminex, DiaSorin S.p.A., and Trivitron Healthcare |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
