Reports
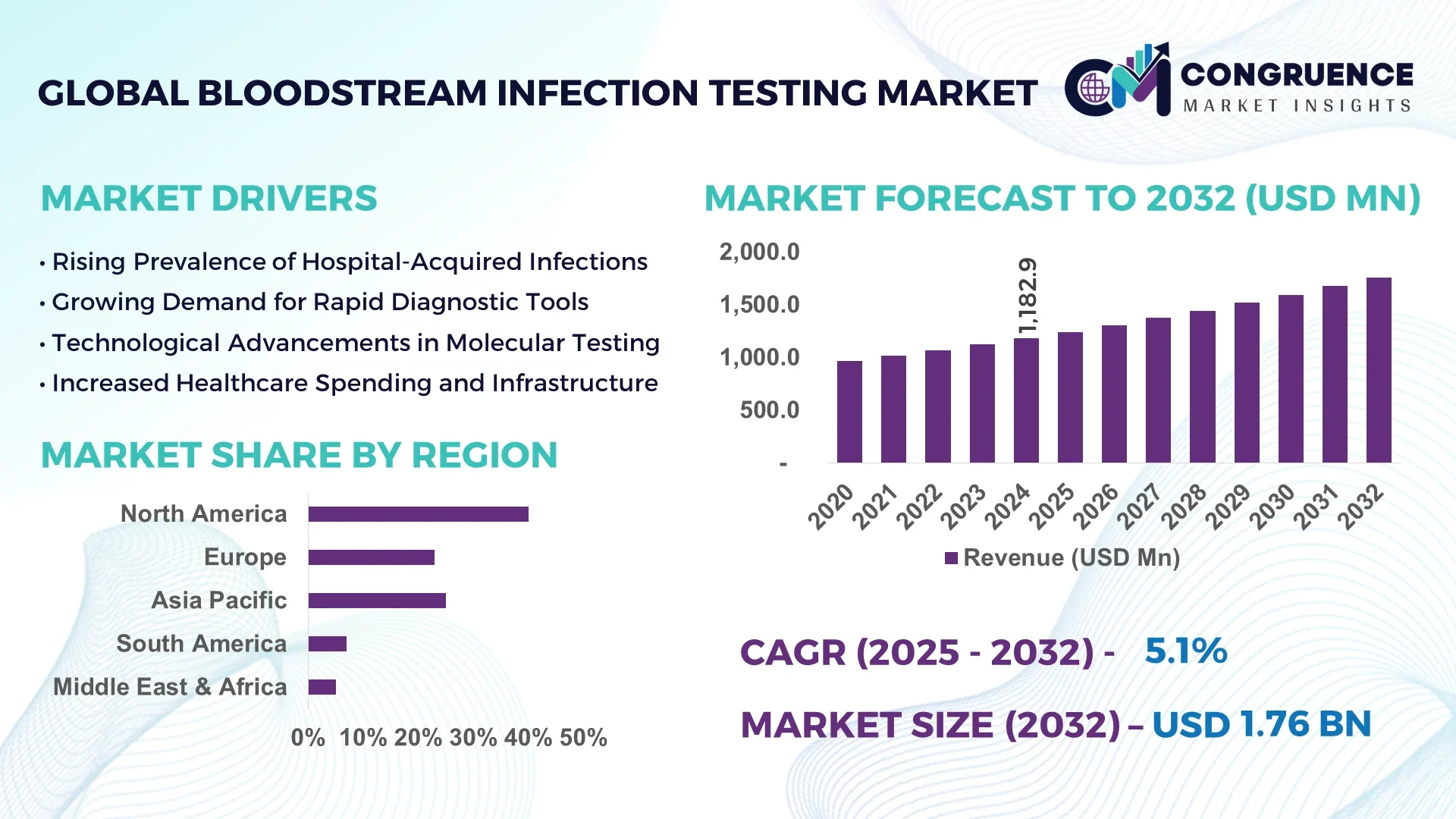
The Global Bloodstream Infection Testing Market was valued at USD 1,182.91 Million in 2024 and is anticipated to reach a value of USD 1,761.06 Million by 2032, expanding at a CAGR of 5.1% between 2025 and 2032.
North America currently leads the bloodstream infection testing market due to the presence of advanced healthcare infrastructure and increasing prevalence of bloodstream infections. In the U.S., hospitals and laboratories are heavily investing in diagnostic technologies, particularly for early-stage bloodstream infection detection, to reduce healthcare-associated risks and improve patient outcomes.
The bloodstream infection testing market is driven by the rising occurrence of bloodstream infections (BSI), which are one of the leading causes of morbidity and mortality globally. Additionally, the growing demand for accurate and rapid diagnostics to identify pathogens such as bacteria and fungi is propelling market growth. Bloodstream infection tests, including blood culture, PCR-based assays, and rapid antigen tests, offer healthcare providers essential tools for diagnosing sepsis and bacteremia. The market is also benefiting from technological advancements, such as automation in laboratory settings and the use of AI for pathogen identification. The surge in hospital-acquired infections, along with increased awareness of infection prevention, has further accelerated the demand for effective testing solutions. Regulatory bodies are also supporting innovation by providing approvals for new diagnostic products, fueling the market's growth trajectory.
Artificial intelligence (AI) is making significant strides in the bloodstream infection testing market, particularly in enhancing the speed, accuracy, and efficiency of diagnostic processes. AI algorithms, specifically machine learning models, are being developed to analyze large datasets, enabling healthcare professionals to make better decisions faster. One of the primary benefits of AI is its ability to improve the accuracy of diagnostic tools, reducing human error and increasing the reliability of test results. For instance, AI-powered systems are helping in the automation of blood culture interpretation, where AI can detect patterns that indicate the presence of pathogens, thus speeding up diagnosis time.
AI is also playing a pivotal role in predictive analytics for bloodstream infections. By analyzing patient history and real-time data, AI can help predict the likelihood of bloodstream infections, enabling preemptive treatments and reducing patient mortality rates. Another area of transformation is AI's involvement in the development of rapid diagnostics, which are essential for managing infections promptly. AI-powered platforms can assess the severity of infections based on diagnostic tests like PCR and genomic sequencing, delivering faster results compared to traditional methods.
Additionally, AI is contributing to the creation of more personalized treatments. By analyzing data from various diagnostic tests, AI systems can help in tailoring individualized treatment plans for patients, ensuring better outcomes. These advancements are revolutionizing not only the laboratory testing but also the overall management of bloodstream infections in clinical settings, improving patient care and reducing healthcare costs.
“In 2024, researchers at a leading medical institute introduced an AI-based diagnostic system that reduced the time to identify bloodstream infections by 40%, significantly enhancing hospital workflows and improving patient care.”
The demand for faster and more reliable diagnostic tests is a significant driver of the bloodstream infection testing market. With the increasing burden of sepsis and bacteremia, healthcare systems are increasingly focused on reducing the time taken to diagnose and treat bloodstream infections. Rapid diagnostic technologies such as PCR, MALDI-TOF MS (matrix-assisted laser desorption ionization time-of-flight mass spectrometry), and next-generation sequencing (NGS) are being adopted to quickly identify pathogens responsible for infections. These technologies are particularly useful in critical care settings, where time-sensitive treatment is crucial to prevent severe outcomes.
Despite the technological advancements in bloodstream infection testing, one of the major restraints is the high cost of certain advanced diagnostic equipment. Technologies like next-generation sequencing and PCR-based assays require significant initial investments, and the associated costs can be a barrier, especially in developing regions where budget constraints are more prevalent. Moreover, these tests often require skilled technicians and specialized laboratory setups, further increasing operational costs. Such factors limit their widespread adoption, especially in low-resource healthcare settings, restricting the overall growth of the market.
There is a growing opportunity for automation in the bloodstream infection testing market. Automation not only reduces the human error factor but also increases throughput, making diagnostic processes more efficient. Automated systems for blood culture testing, pathogen identification, and antimicrobial susceptibility testing are gaining popularity. These systems can process a larger number of samples at faster speeds, improving testing accuracy and allowing healthcare providers to manage high patient volumes. The demand for automated solutions is further driven by the need to optimize healthcare resources and reduce operational costs, presenting a significant opportunity for market players to invest in such technologies.
One of the most prominent challenges facing the bloodstream infection testing market is the shortage of skilled professionals, such as microbiologists and lab technicians, who are needed to operate advanced diagnostic equipment. Bloodstream infection testing, especially when utilizing complex technologies like PCR or sequencing, requires a high level of expertise to interpret results accurately. The global shortage of skilled laboratory personnel is exacerbated in regions with limited healthcare infrastructure, potentially leading to delays in diagnostic results and treatment. This challenge hinders the effective implementation of cutting-edge diagnostic solutions and impacts the overall efficiency of bloodstream infection testing processes.
• Rise in Automation and Rapid Diagnostics: The increasing adoption of automated diagnostic systems is reshaping the demand dynamics in the bloodstream infection testing market. Automated blood culture systems and molecular diagnostic platforms are being increasingly utilized to enhance the speed and accuracy of pathogen identification. These systems are significantly reducing manual errors and improving throughput in high-volume hospital labs, particularly in regions like North America and Europe. The surge in demand for rapid diagnostics to address sepsis and bacteremia is driving this trend, enabling quicker clinical decisions and better patient outcomes.
• Integration of Artificial Intelligence (AI): AI integration in bloodstream infection testing is revolutionizing the market by enhancing diagnostic accuracy and reducing processing time. AI-driven algorithms can analyze complex microbiological data and predict pathogen resistance, significantly improving the effectiveness of blood culture analysis. These technologies are particularly valuable in critical care environments where fast decision-making is essential. The use of AI is growing rapidly, with significant investments being made in the development of intelligent systems to aid in early detection of infections, particularly in hospitals and healthcare centers in developed regions.
• Shift Towards Point-of-Care Testing (POCT): Point-of-care testing is gaining traction in the bloodstream infection testing market as it provides rapid, accurate results outside of traditional laboratory settings. This trend is driven by the need for faster diagnoses in emergency care, intensive care units, and ambulatory settings. POCT devices for detecting bloodstream infections, including portable PCR systems and rapid antigen tests, are becoming more common, allowing clinicians to make immediate treatment decisions, particularly in rural and remote areas where access to large labs may be limited.
• Increase in Government and Healthcare Investments: Governments and healthcare organizations are increasingly investing in advanced diagnostic technologies to tackle the growing burden of bloodstream infections, including sepsis and bacteremia. These investments are aimed at improving diagnostic capabilities in hospitals, enhancing infection control measures, and reducing healthcare-associated risks. Particularly in emerging markets, governments are focusing on strengthening healthcare infrastructure and boosting the adoption of state-of-the-art testing systems to combat the rising rates of infections. Such trends are expected to further fuel the demand for advanced testing solutions in the coming years.
The bloodstream infection testing market can be segmented based on type, application, and end-user insights. By type, the market is divided into blood culture, molecular diagnostic tests, and other rapid diagnostic tests. In terms of application, the market includes hospital labs, diagnostic centers, and research laboratories. End-user insights reveal that hospitals dominate the market, followed by diagnostic centers and research laboratories. These segments provide critical insights into the demand dynamics, identifying areas where growth is concentrated and understanding the varying needs of each user group.
The bloodstream infection testing market is primarily categorized into blood culture tests, molecular diagnostic tests, and rapid diagnostic tests. Blood culture tests are currently the leading segment, as they have been the standard for diagnosing bloodstream infections for decades. They are crucial for identifying the specific pathogens responsible for infections, making them indispensable in hospital settings. However, molecular diagnostic tests are the fastest-growing segment due to their ability to provide quicker and more accurate results, especially for detecting antibiotic resistance. PCR-based assays and next-generation sequencing are revolutionizing pathogen identification and accelerating diagnosis. Rapid diagnostic tests are also gaining traction, especially in emergency and point-of-care settings, as they provide quick results, enabling faster treatment decisions. Molecular diagnostics are expected to grow rapidly as healthcare providers seek advanced, more reliable diagnostic tools to combat the rise of multidrug-resistant infections.
The bloodstream infection testing market is divided into several key applications, including hospital labs, diagnostic centers, and research laboratories. Hospital laboratories currently lead the market due to the critical role they play in diagnosing sepsis and bacteremia, particularly in intensive care units and emergency departments. The demand for faster and more accurate diagnostic tools is driving growth in this segment. Diagnostic centers are also experiencing growth, particularly in urban areas, as these centers offer specialized diagnostic services and cater to patients who require rapid testing. The research laboratory segment, although smaller, is growing steadily as advancements in pathogen detection and antimicrobial resistance research continue to evolve. Diagnostic centers, with their increased focus on rapid testing solutions, are likely to see the highest growth in the coming years due to their ability to provide fast, reliable results outside traditional hospital settings.
In terms of end-users, the bloodstream infection testing market is segmented into hospitals, diagnostic centers, and research laboratories. Hospitals are the dominant end-user segment, accounting for the largest share of the market. Hospitals are the primary settings for the diagnosis and treatment of bloodstream infections, particularly in emergency and critical care departments. The demand for advanced diagnostic tools in hospitals is driving the market's growth, with hospitals continually investing in state-of-the-art diagnostic technologies. Diagnostic centers are the fastest-growing end-user segment, driven by the increasing demand for rapid diagnostics and testing outside of hospital settings. These centers are playing an important role in reducing hospital congestion and providing timely diagnostic results, particularly for outpatient care. Research laboratories are important but represent a smaller portion of the market. They contribute to advancements in diagnostic methods, particularly in the research of new pathogens and antimicrobial resistance patterns. Research labs are expected to see moderate growth as they continue to innovate and refine diagnostic technologies.
North America accounted for the largest market share at 40% in 2024, however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 6.3% between 2025 and 2032.
North America led the market in 2024 due to advanced healthcare infrastructure and the growing prevalence of bloodstream infections. The region’s market is expected to continue holding a dominant position due to its significant investments in healthcare technologies. On the other hand, Asia-Pacific is experiencing rapid growth due to rising healthcare demands, improved medical infrastructure, and increasing awareness of diagnostic technologies. These trends are expected to shape the market’s future trajectory, with particular growth in emerging economies.
Rising Adoption of Rapid Diagnostics Across U.S. Healthcare Systems
North America holds the largest share of the bloodstream infection testing market, accounting for nearly 40% of the global market in 2024. The United States dominates this region due to its highly advanced healthcare system and strong emphasis on early diagnosis and treatment of infections, especially in hospital settings. The region's high demand for rapid diagnostic tools, particularly for sepsis and bacteremia, is driving the market. Canada also contributes significantly to the market with increasing investments in healthcare infrastructure and rising adoption of advanced diagnostic solutions. Hospitals in these regions continue to integrate innovative diagnostic systems to improve infection control measures, fueling the demand for blood culture and molecular diagnostic tests.
Increased Focus on AMR Control Driving Market Expansion
Europe holds a substantial share in the bloodstream infection testing market, with countries like Germany, France, and the United Kingdom leading the way. The demand for advanced diagnostics in critical care and emergency departments is one of the key factors contributing to the market’s growth. Moreover, Europe’s robust healthcare framework supports the integration of modern diagnostic technologies, such as molecular testing and AI-driven diagnostics. The rising incidence of hospital-acquired infections and an increasing aging population are also driving the need for enhanced diagnostic solutions in European hospitals. As a result, the demand for rapid and accurate testing methods continues to expand in the region.
Growing Investments in Hospital Infrastructure and Diagnostic Innovation
The Asia-Pacific region is witnessing the fastest growth in the bloodstream infection testing market, expected to see significant expansion between 2025 and 2032. Countries like China and India are driving this growth due to their rapidly improving healthcare infrastructure and increasing healthcare expenditure. The rising burden of infectious diseases, coupled with growing healthcare awareness, is leading to greater adoption of advanced diagnostic technologies. Additionally, with the increasing number of healthcare facilities adopting molecular diagnostic and rapid test systems, the region is experiencing a shift towards faster and more accurate infection detection. The Asia-Pacific market is expected to continue its growth, particularly with the rise in sepsis awareness programs in the region.
Public Health Campaigns Fueling Diagnostic Awareness in South America
The bloodstream infection testing market in South America is evolving with growing demand for diagnostic tests driven by an increase in the region’s healthcare investments. Brazil and Argentina are the leading markets in South America, with hospitals and diagnostic centers increasingly adopting advanced testing technologies. The rising incidence of hospital-acquired infections and the need for quicker, more accurate diagnostic solutions are accelerating the demand for blood culture and molecular diagnostic tests. Although the market in South America is still in a developing phase, the increasing adoption of point-of-care testing devices is expected to support significant growth in the coming years.
Strengthening Laboratory Capacity Amid Rising Sepsis Cases
In the Middle East and Africa, the bloodstream infection testing market is gaining momentum due to rising investments in healthcare infrastructure and the growing prevalence of infectious diseases. Countries like Saudi Arabia and South Africa are taking the lead in adopting advanced diagnostic technologies in hospitals and diagnostic centers. The market is also supported by healthcare initiatives aimed at improving infection prevention and control measures, which are driving the demand for rapid and accurate infection tests. Furthermore, governments in the region are focusing on expanding access to advanced healthcare services, boosting the demand for effective bloodstream infection testing solutions.
The bloodstream infection testing market is highly competitive, driven by increasing global awareness of hospital-acquired infections and the growing demand for early diagnostic solutions. Key players are focused on product innovation, expansion of diagnostic test menus, and the integration of automation to reduce manual errors in laboratories. Leading companies are offering advanced blood culture systems, nucleic acid amplification tests, and rapid PCR-based identification methods.
The competition has intensified with companies introducing multiplex assays capable of identifying multiple pathogens in a single test. Strategic collaborations with hospitals and diagnostic labs are becoming more common, enhancing market presence and customer reach. Additionally, players are entering emerging economies with cost-effective products tailored to local infrastructure. For example, several companies have increased production capacities in Asia-Pacific and Latin America to meet growing demands. The launch of next-generation sequencing-based infection diagnostics is also gaining traction. With rising healthcare investments, the market is likely to witness even greater product differentiation and strategic alliances in the coming years.
bioMérieux SA
F. Hoffmann-La Roche AG
BD (Becton, Dickinson and Company)
Thermo Fisher Scientific Inc.
Luminex Corporation
Accelerate Diagnostics Inc.
Bruker Corporation
Siemens Healthineers
QuidelOrtho Corporation
Abbott Laboratories
Qiagen NV
Nanosphere Inc.
Technological advancements are significantly reshaping the bloodstream infection testing market, with innovations focused on speed, accuracy, and automation. Automated blood culture systems have improved pathogen detection times from over 72 hours to as little as 24 hours. Modern diagnostics increasingly use molecular techniques, such as polymerase chain reaction (PCR), which allow for rapid identification of infectious agents directly from blood samples, bypassing lengthy culturing steps.
Artificial intelligence and machine learning are now being integrated into laboratory workflows to interpret complex data from blood tests, improving diagnosis and reducing human error. The adoption of MALDI-TOF mass spectrometry has enhanced microorganism identification, allowing labs to analyze thousands of samples per day with high precision. Moreover, point-of-care testing kits with lateral flow immunoassay technology are making testing accessible even in remote regions, thereby expanding the patient pool and improving healthcare reach.
Next-generation sequencing (NGS) is also emerging as a powerful tool for detecting rare and resistant pathogens. It allows clinicians to obtain a comprehensive microbial profile in a single test run. Furthermore, multiplex PCR panels are now capable of detecting more than 20 bloodstream pathogens simultaneously, offering clinicians faster and broader diagnostic coverage. These technologies are not only increasing diagnostic speed and sensitivity but also facilitating better-targeted antimicrobial therapy, improving patient outcomes.
In June 2024, the U.S. Food and Drug Administration (FDA) issued an alert regarding a shortage of Becton Dickinson's blood test tubes, essential for diagnosing bacterial and fungal infections. The agency urged healthcare providers to prioritize high-risk patients, such as those exhibiting signs of bloodstream infections, due to the potential impact on diagnosis and treatment. Becton Dickinson attributed the shortage to reduced availability of plastic bottles from a supplier and implemented measures like air shipments and modified manufacturing schedules to address the issue.
In 2023, Korean researchers developed a groundbreaking sepsis test capable of identifying infection-causing species within hours, achieving a 100% match rate in identification. This ultra-rapid antimicrobial susceptibility testing method detects drug-resistant pathogens directly from whole blood without separation, significantly reducing the testing time compared to the traditional 2-3 days required for blood culture samples. Initial tests on 190 patients showed an average turnaround of 13 hours, promising to decrease sepsis fatalities and reduce unnecessary antibiotic use.
In 2023, scientists introduced a novel testing technology poised to revolutionize sepsis treatment by reducing the time to identify effective antibiotics from the current two days to approximately 12 hours. This method employs peptide molecules attached to magnetic nanoparticles that bind to bacteria in a blood sample, eliminating the need for time-consuming culture steps. The bacteria's DNA is then amplified and analyzed to identify the microbes, while an AI system examines bacterial growth patterns to determine effective antibiotics. This approach aims to cut antimicrobial susceptibility testing time by 30-40 hours and could be clinic-ready within two to three years.
In August 2022, Immunexpress Pty Ltd., an Australian molecular diagnostics company, launched their SeptiCyte RAPID cartridges. These cartridges utilize undiluted ethylenediaminetetraacetic acid (EDTA) blood as a validated sample type, enhancing the precision and speed of sepsis diagnosis. This advancement paves the way for broader and quicker clinical implementation of rapid diagnostic tests for bloodstream infections.
The Bloodstream Infection Testing Market encompasses a wide range of diagnostic tools and technologies aimed at identifying pathogens in the bloodstream, crucial for the timely treatment of infections like sepsis. The market includes various products such as reagents, consumables, instruments, and software solutions utilized in hospitals, diagnostic laboratories, and research institutions.
Key technologies in this market include blood culture tests, polymerase chain reaction (PCR), mass spectrometry, and next-generation sequencing (NGS), each offering varying degrees of speed and accuracy in pathogen detection. The integration of artificial intelligence and machine learning is also enhancing diagnostic capabilities, enabling faster and more precise identification of bloodstream infections.
The market is segmented by product type, technology, sample type, end-user, and region, providing a comprehensive analysis of trends and growth opportunities. Factors driving market growth include the increasing prevalence of hospital-acquired infections, rising antimicrobial resistance, and the need for rapid diagnostic solutions. Challenges such as high costs of advanced testing methods and limited access in low-resource settings are also addressed in the report.
Overall, the Bloodstream Infection Testing Market Report offers an in-depth examination of current market dynamics, technological advancements, and future prospects, serving as a valuable resource for stakeholders aiming to understand and navigate the complexities of this critical healthcare sector.
| Report Attribute/Metric | Report Details |
|---|---|
|
Market Revenue in 2024 |
USD 1,182.91 Million |
|
Market Revenue in 2032 |
USD 1,761.06 Million |
|
CAGR (2025 - 2032) |
5.1% |
|
Base Year |
2024 |
|
Forecast Period |
2025 - 2032 |
|
Historic Period |
2020 - 2024 |
|
Segments Covered |
By Types
By Application
By End-User
|
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Region Covered |
North America, Europe, Asia-Pacific, South America, Middle East, Africa |
|
Key Players Analyzed |
bioMérieux SA, F. Hoffmann-La Roche AG, BD (Becton, Dickinson and Company), Thermo Fisher Scientific Inc., Luminex Corporation, Accelerate Diagnostics Inc., Bruker Corporation, Siemens Healthineers, QuidelOrtho Corporation, Abbott Laboratories, Qiagen NV, Nanosphere Inc. |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
