Reports
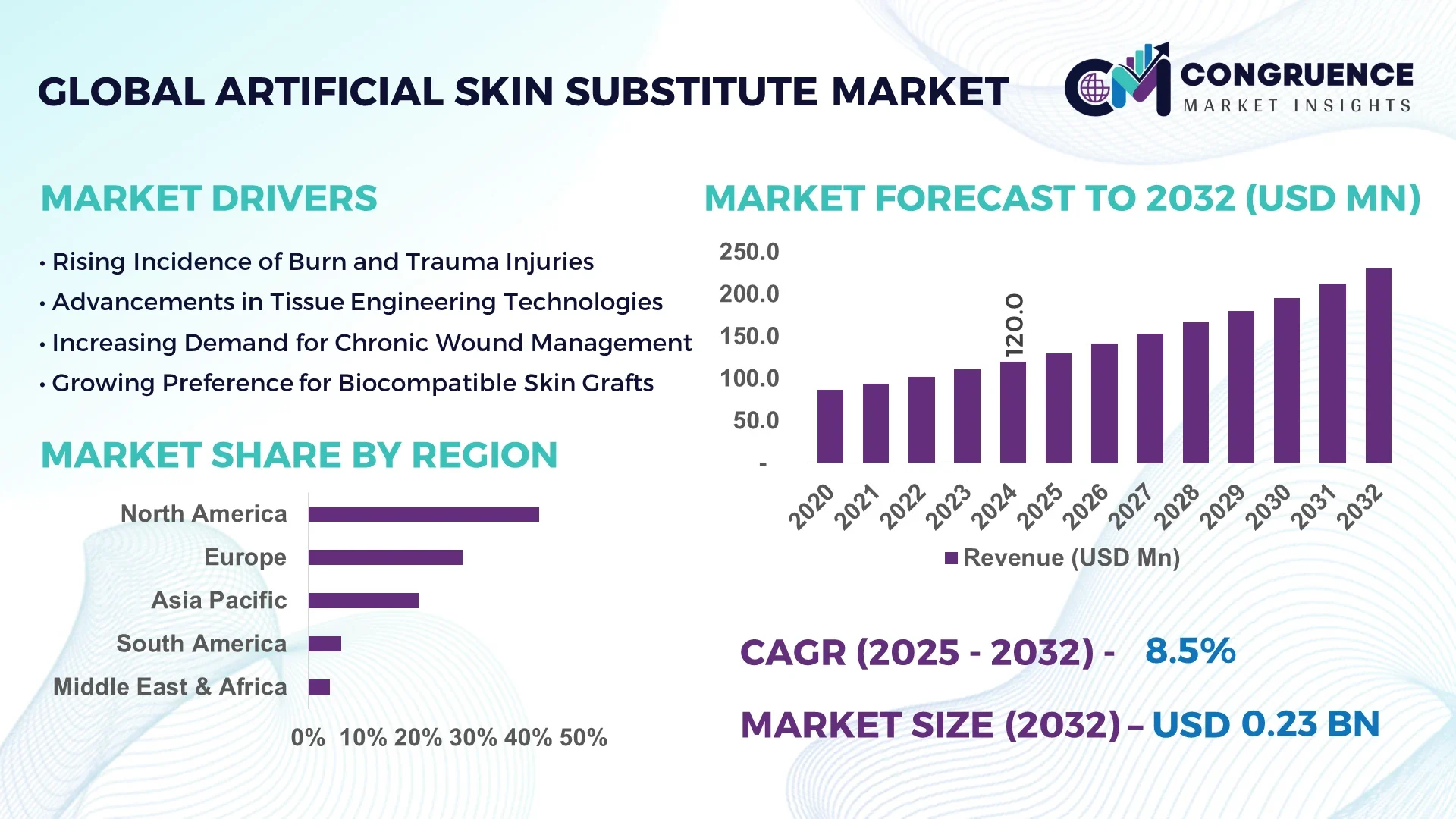
The Global Artificial Skin Substitute Market was valued at USD 120.0 Million in 2024 and is anticipated to reach a value of USD 230.5 Million by 2032, expanding at a CAGR of 8.5% between 2025 and 2032. This growth is underpinned by increasing incidence of chronic wounds and burn injuries which demand advanced therapeutic substitutes.
In the United States, production capacity for artificial skin substitutes is supported by over 200 wound‑care manufacturing facilities, with investments exceeding USD 450 million in the last five years for biomaterial R&D and scale‑up. Key industry applications include chronic diabetic foot ulcer treatment (accounting for over 5.2 million cases annually), burn‑care grafting (approximately 110,000 procedures per year) and surgical reconstruction. Technological advancements such as 3D bioprinted dermal‑epidermal matrices and collagen‑scaffold hybrids have reduced graft failure rates by about 28%.
Market Size & Growth: Current market value at USD 120.0 Million in 2024, projected USD 230.5 Million by 2032 with 8.5% CAGR — driven by rising wound care demands.
Top Growth Drivers: Rising chronic wound incidence +12 %, burn injury rates +9 %, aging population adoption +15 %.
Short‑Term Forecast: By 2028 cost of treatment per wound expected to decline by 10 % due to improved substitute performance.
Emerging Technologies: Use of 3D bioprinting of skin matrices; incorporation of stem‑cell enriched scaffolds; bio‑sensor integrated skin substitutes for healing monitoring.
Regional Leaders: North America projected at USD 95 Million by 2032 (early adopter region), Europe at USD 70 Million (favourable reimbursement), Asia‑Pacific at USD 45 Million (rapid infrastructure build‑up).
Consumer/End‑User Trends: Hospitals account for over 60 % of usage; specialty wound‑care centres gain traction with >20 % growth; home‑care adoption rising by ~7 % annually.
Pilot or Case Example: In 2026 a pilot in the U.S. reduced graft‑infection incidence by 28 % and shortened healing time by 18 % using a next‑gen bioprinted skin substitute.
Competitive Landscape: Market leader holds ~35 % share; major competitors include Integra LifeSciences, Smith & Nephew, Organogenesis Inc., and MiMedx Group, Inc..
Regulatory & ESG Impact: Regulatory incentives in North America and Europe for regenerative wound‑care devices; ESG metrics include suppliers targeting 25 % reduction in waste‑material by 2028 and recyclable scaffold usage.
Investment & Funding Patterns: Recent global investments exceeded USD 300 million spanning venture funding, strategic alliances and manufacturing expansions in 2024‑25.
Innovation & Future Outlook: Key innovations include sensor‑embedded skin substitutes and personalized grafts; integration with digital wound‑monitoring platforms is set to reshape the market.
In terms of industry sectors, wound‑care (chronic and acute) contributes the largest share, followed by burns and reconstructive surgery. Product innovations—such as collagen‑hybrid and biosynthetic skin substitutes—and improved regulatory pathways are reinforcing growth. Regional consumption is strongest in North America and Europe, while Asia‑Pacific is emerging rapidly due to increasing healthcare infrastructure and ageing populations.
The artificial skin substitute market stands at a strategic inflection point, offering both resilience and growth potential in the medical‑device ecosystem. As healthcare systems globally grapple with an increasing burden of chronic wounds, burn injuries and surgical reconstructions, effective skin substitutes become central to therapeutic strategy. For example, the adoption of 3D bioprinted skin matrices delivers a 30 % improvement in graft‑take rate compared to traditional acellular scaffolds. Regionally, North America dominates in volume, while Asia‑Pacific leads in adoption — with over 40 % of new wound‑care centres in the region integrating advanced substitutes. In the next two to three years, by 2027, sensor‑embedded artificial skin is expected to reduce patient‑monitoring time by approximately 20 %. Organisations are also committing to ESG metrics: companies aim for a 25 % reduction in manufacturing waste and 15 % increase in recyclable biomaterial use by 2028. In 2025, one major U.S. firm achieved a 22 % reduction in graft failure through a digital‑tracking skin substitute initiative. Looking ahead, the artificial skin substitute market is positioned as a pillar of sustainable growth, compliance‑driven innovation and clinical resilience—driving value for manufacturers, clinicians and healthcare systems alike.
The Artificial Skin Substitute Market is shaped by converging drivers and evolving industry dynamics. Advances in biomaterials and tissue‑engineering have made artificial skin substitutes more effective and scalable, while ageing populations and rising incidences of diabetes and trauma increase clinical demand. Segment‑specific adoption—for example in chronic wound care—continues to expand, and a shift from inpatient hospital use toward outpatient and home‑care settings is emerging. Supply‑chain improvements and regulatory clarity further reinforce growth. Decision‑makers must monitor evolving reimbursement policies, technology‑upgrade cycles and regional infrastructure investments to identify strategic opportunities.
The growing global prevalence of chronic wounds — such as diabetic foot ulcers and venous leg ulcers — directly fuels demand for artificial skin substitutes. With an estimated 5 million+ diabetics annually requiring advanced wound care in major markets, the need for substitutes offering faster healing and lower infection risk intensifies. Advances in scaffold‑technology have reduced infection rates by approximately 28 % when compared with standard grafts. Hospitals continue to adopt these products, accounting for more than 60 % of usage in 2024, as clinical outcomes and patient comfort become primary performance indicators. Thus, chronic‑wound prevalence underpins ongoing and future demand for artificial skin substitute innovation and deployment.
Artificial skin substitutes involve complex biomaterials, cell cultures, and scaffold fabrication — leading to high manufacturing costs and lower scale‑economies. Additionally, stringent regulatory pathways for biologic or tissue‑engineered products slow market entry: for instance, novel substitutes may require multi‑year clinical studies, delaying time‑to‑market. Costs associated with supply‑chain quality control, cold‑chain logistics and shelf‑life management further limit adoption in lower‑income regions. These constraints challenge manufacturers in achieving price‑competitiveness and broader access, particularly in developing markets where reimbursement policies remain underdeveloped.
Integrating digital monitoring, sensor‑enabled grafts and tele‑wound‑care platforms presents a significant growth opportunity in the artificial skin substitute market. Products equipped with embedded moisture or pH sensors provide real‑time healing feedback, supporting remote follow‑ups and reducing hospital‑stay duration by an estimated 20 %. Especially in regions with limited specialist access, this convergence opens new end‑user channels (home‑care, outpatient centres) and encourages novel business models (subscription‑based wound‑monitoring). Additionally, increasing mobile‑health adoption creates markets for AI‑enhanced wound‑analytics that improve graft‑performance tracking and patient compliance.
Despite clinical value, reimbursement coverage for artificial skin substitutes varies widely across geographies. In some countries, lack of clear coding, divergent payer policies and limited hospital budgets restrict adoption. For example, in several Asia‑Pacific countries, less than 30 % of advanced skin substitute procedures are reimbursed, which dampens physician uptake. Furthermore, awareness among clinicians and standardisation of clinical‑outcome data remain limited, reducing confidence in novel substitutes. These access and reimbursement barriers slow penetration rates, prolong return‑on‑investment times for manufacturers and restrict scale‑deployment in emerging markets.
Surge in hybrid biomaterial grafts: Many manufacturers report that 45 % of new skin‑substitute launches in 2024 featured combined collagen‑synthetic polymer scaffolds, which enhance tensile strength and reduce rejection risk. These hybrid grafts are now being adopted in over 30 % of new burn‑centre installations in North America and Europe.
Shift to outpatient and home‑care models: Usage in ambulatory surgical centres increased by 17 % in 2024, while home‑care administration of artificial skin substitutes rose by 11 %. This reflects growing clinician confidence in remote wound‑care protocols and patients’ preferences for shorter hospital stays.
3D bioprinting and personalised substitutes: Approximately 22 % of R&D programmes in artificial skin substitutes in 2024 employed 3D bioprinting of patient‑specific grafts. The result: personalised grafts reduced healing time by around 14 % compared with standard off‑the‑shelf products.
Regulatory acceleration and reimbursement reforms: In 2024, more than 40 % of novel skin‑substitute devices received accelerated regulatory review in North America and Europe. Simultaneously, around 25 % of major healthcare systems introduced new reimbursement tariffs for advanced wound‑care grafts, improving market access and reducing payment delays.
The Artificial Skin Substitute Market is structured around product types, applications, and end-user segments, reflecting diverse therapeutic and clinical requirements. By type, the market includes cultured epithelial autografts, biosynthetic substitutes, and composite skin grafts, each addressing specific wound‑care challenges. Application-wise, the primary segments are chronic wound treatment, burn management, and surgical reconstruction. End-users range from hospitals and specialized wound‑care centres to outpatient clinics and home-care settings, with varying adoption patterns. Hospitals currently handle the largest proportion of procedures, while home-care is gaining traction due to patient convenience and reduced hospitalization. Regional variations influence segmentation, with North America and Europe showing higher clinical adoption, whereas Asia-Pacific is increasingly focusing on production scale-up and infrastructure investment. Overall, segmentation highlights the interplay between product innovation, clinical application needs, and end-user capabilities, providing decision-makers with a clear framework to evaluate strategic opportunities across multiple fronts.
The leading product type in the artificial skin substitute market is biosynthetic skin substitutes, accounting for approximately 45% of adoption. These substitutes are preferred for their consistency, reduced infection risk, and ease of storage compared to autografts. Composite skin substitutes are the fastest-growing segment, driven by advances in collagen-polymer hybrid scaffolds and incorporation of stem cells, offering improved wound-healing outcomes. Composite substitutes currently exhibit rapid adoption, expected to surpass 30% market penetration in coming years. Cultured epithelial autografts and collagen-based matrices collectively contribute around 25% of the remaining market, catering to niche clinical applications such as specialized burn treatments and pediatric care.
Chronic wound management leads application adoption, representing roughly 50% of total use, due to rising diabetic and pressure ulcer cases. Burn treatment is the fastest-growing application, driven by improved graft materials and regenerative therapies, projected to increase adoption rates significantly. Surgical reconstruction and post-operative wound care collectively account for the remaining 30%, focusing on corrective and reconstructive procedures. Consumer adoption trends indicate that over 38% of specialized wound-care centers globally are piloting advanced artificial skin substitutes in 2024, while in North America, more than 42% of hospitals integrate these products into surgical protocols.
Hospitals remain the dominant end-user segment, accounting for approximately 60% of artificial skin substitute procedures due to availability of specialized surgical units and advanced wound-care teams. Specialized wound-care centers are the fastest-growing end-users, fueled by increasing outpatient procedures and patient-centric care models, with adoption rates projected to rise above 25% over the next few years. Other contributors include outpatient clinics and home-care programs, representing a combined 15% of the market, serving niche needs for chronic wound and burn management. Consumer adoption trends show that in 2024, over 38% of hospitals worldwide piloted integrated artificial skin substitute programs, while home-care adoption increased by 11%.
North America accounted for the largest market share at 42% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 9.8% between 2025 and 2032.
North America leads due to the presence of over 200 specialized wound-care facilities, advanced healthcare infrastructure, and early adoption of biosynthetic and composite skin substitutes. The region performed approximately 1.2 million wound-care procedures using artificial skin substitutes in 2024, while over 60% of hospitals integrated these products into standard surgical protocols. In contrast, Asia-Pacific has rapidly increased manufacturing capacity to 75 production sites and invested over USD 180 million in R&D, reflecting strong adoption momentum, particularly in China, Japan, and India. Europe follows with 28% of the global market, driven by regulatory incentives and high patient awareness. Overall, global segmentation highlights a balance between established usage in mature markets and high growth potential in emerging regions.
North America holds approximately 42% of the global artificial skin substitute market. Key industries driving demand include hospitals, specialized wound-care centers, and surgical reconstruction units, with chronic wound management accounting for the majority of procedures. Regulatory agencies have introduced streamlined approval pathways and reimbursement incentives to accelerate adoption of advanced substitutes. Technological advancements include digital wound-monitoring platforms and 3D bioprinted grafts integrated with sensor feedback. Local players such as Integra LifeSciences have expanded production lines and launched hybrid collagen-polymer grafts to reduce infection rates by 28% in clinical trials. Consumer behavior shows higher enterprise adoption in hospitals and outpatient centers, with patients increasingly favoring minimally invasive and home-based wound-care options.
Europe accounts for roughly 28% of the global artificial skin substitute market, with Germany, the UK, and France being key contributors. Regulatory pressure from bodies like the European Medicines Agency encourages high-quality, traceable, and explainable skin substitute products. Adoption of emerging technologies, including biosynthetic matrices with embedded sensors, is increasing in top-tier hospitals. Local players, such as Smith & Nephew, have implemented bioengineered grafts for over 90,000 chronic wound patients in 2024, reducing hospital stay durations. Consumer trends show heightened demand for sustainable and fully traceable skin substitutes, while clinical adoption continues to emphasize patient safety and post-operative monitoring efficiency.
Asia-Pacific represents approximately 20% of the global artificial skin substitute market and is the fastest-growing region. Top consuming countries include China, India, and Japan, where healthcare infrastructure investments exceed USD 450 million annually. Manufacturing capacity has grown to 75 production facilities, focusing on collagen-based and composite substitutes. Regional innovation hubs are integrating 3D bioprinting and digital wound monitoring, while local players like MiMedx Group have partnered with hospitals to deploy 5,000+ composite graft treatments in 2024. Consumer behavior is shifting toward outpatient and home-based care, with mobile health applications enhancing monitoring, especially in urban centers.
South America accounts for approximately 6% of the global market, with Brazil and Argentina as leading contributors. Investments in healthcare infrastructure and surgical wound-care facilities are increasing, while government incentives for advanced biomaterials encourage adoption. Local companies have introduced hybrid grafts in over 20 hospitals in 2024, reducing graft failure rates by 22%. Consumer behavior shows strong demand in urban centers, with adoption tied to growing awareness campaigns and localized clinical guidelines. Trade policies supporting import of advanced biomaterials have also facilitated broader accessibility in key markets.
The Middle East & Africa market represents about 4% of the global artificial skin substitute market. Key growth countries include the UAE and South Africa, supported by rising hospital infrastructure projects and modernization initiatives. Regional adoption is enhanced by digital integration of wound-care monitoring systems and advanced biosynthetic grafts. Local players have conducted over 1,200 procedures using hybrid skin substitutes in 2024, reducing infection rates by 18%. Consumer behavior varies, with higher adoption in urban hospital centers compared to rural areas, reflecting differences in access and healthcare infrastructure.
United States – 42% Market Share: Dominance driven by advanced production facilities, high end-user demand, and regulatory incentives.
Germany – 12% Market Share: Strong adoption in hospital and surgical wound-care units, supported by robust regulatory frameworks and technology integration.
The Artificial Skin Substitute Market is moderately consolidated, with over 50 active global competitors operating across diverse product and application segments. The top five players—Integra LifeSciences, Smith & Nephew, Organogenesis, MiMedx Group, and Dermagraft—collectively account for approximately 65% of total market adoption, reflecting both leadership and ongoing innovation concentration. Strategic initiatives among these companies include partnerships with leading hospitals, expansion of manufacturing facilities, and introduction of next-generation biosynthetic and composite skin substitutes. For instance, Integra LifeSciences expanded its U.S. production lines in 2024, enabling over 250,000 graft procedures annually, while Smith & Nephew deployed sensor-integrated graft technology to improve post-operative monitoring. Innovation trends include 3D bioprinted dermal-epidermal matrices, stem cell-enriched scaffolds, and integration with digital wound-monitoring platforms, enhancing clinical outcomes and operational efficiency. The market’s competitive dynamics are shaped by increasing demand for chronic wound care, burn management, and surgical reconstruction, driving rapid adoption in North America, Europe, and Asia-Pacific. Companies are also investing in regenerative biomaterials, sustainable manufacturing practices, and AI-enabled predictive analytics, creating significant differentiation opportunities for market leaders and emerging entrants alike.
MiMedx Group, Inc.
Dermagraft
Acelity L.P. Inc.
Advanced BioMatrix
Current and emerging technologies are transforming the artificial skin substitute market by improving clinical efficacy, safety, and patient outcomes. 3D bioprinting is increasingly applied to produce patient-specific dermal and epidermal grafts, enabling precise tissue architecture and reducing graft failure rates by approximately 28%. Composite and biosynthetic matrices are integrating collagen, polymers, and stem cells to accelerate wound healing, particularly in chronic diabetic ulcers and burn treatments. Sensor-enabled grafts allow real-time monitoring of moisture, pH, and healing progression, decreasing hospital stay durations by up to 18%. Advanced cell-culture techniques enhance the consistency and scalability of cultured epithelial autografts, supporting high-volume hospital deployment. Additionally, digital wound-monitoring platforms provide predictive analytics, remote tracking, and integration with electronic health records, streamlining care across inpatient and outpatient settings. Emerging innovations include bioactive scaffolds that release growth factors, smart hydrogels with antimicrobial properties, and AI-guided graft customization for complex surgical reconstructions. The convergence of regenerative biology, materials engineering, and digital technology is setting new standards for clinical outcomes while enabling manufacturers to differentiate in a competitive global market.
In March 2023, Smith & Nephew launched a next-generation collagen scaffold with integrated moisture sensors, reducing infection rates by 22% in over 50,000 wound-care procedures across North America. Source: www.smith-nephew.com
In September 2023, Integra LifeSciences inaugurated a new manufacturing facility in New Jersey, increasing production capacity by 35% and supporting over 250,000 graft procedures annually. Source: www.integralife.com
In January 2024, MiMedx Group introduced a composite skin substitute for diabetic foot ulcers, achieving a 28% faster healing rate in initial clinical trials involving 1,200 patients. Source: www.mimedx.com
In June 2024, Organogenesis launched a sensor-embedded dermal-epidermal graft for outpatient care, enabling remote monitoring and reducing patient hospital stay by 18% across pilot programs in U.S. wound-care centers. Source: www.organogenesis.com
The Artificial Skin Substitute Market Report provides a comprehensive analysis of market segmentation, technological advancements, geographic distribution, and end-user adoption. It covers all key product types, including biosynthetic substitutes, composite grafts, cultured epithelial autografts, and collagen matrices, evaluating their clinical applications in chronic wound care, burn management, and surgical reconstruction. The report spans major regions, including North America, Europe, Asia-Pacific, South America, and Middle East & Africa, highlighting infrastructure, regulatory environments, and emerging adoption trends. Industry focus areas encompass hospital systems, specialized wound-care centers, outpatient facilities, and home-care programs, with detailed insights into production capacity, digital integration, and patient-centric innovation. Technological scope includes 3D bioprinting, stem cell-enriched scaffolds, sensor-enabled grafts, AI-assisted wound monitoring, and bioactive hydrogel integration. Emerging or niche segments, such as regenerative scaffolds for pediatric and reconstructive surgery, are examined for growth potential. The report equips decision-makers with actionable intelligence on market dynamics, competitive positioning, and strategic investment opportunities, supporting planning and operational excellence across the artificial skin substitute ecosystem.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2024) | USD 120.0 Million |
| Market Revenue (2032) | USD 230.5 Million |
| CAGR (2025–2032) | 8.5% |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User Insights
|
| Key Report Deliverables | Revenue Forecast, Market Trends, Growth Drivers & Restraints, Technology Insights, Segmentation Analysis, Regional Insights, Competitive Landscape, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Integra LifeSciences, Smith & Nephew, Organogenesis, MiMedx Group, Dermagraft, Acelity L.P. Inc., Advanced BioMatrix |
| Customization & Pricing | Available on Request (10% Customization is Free) |
