Reports
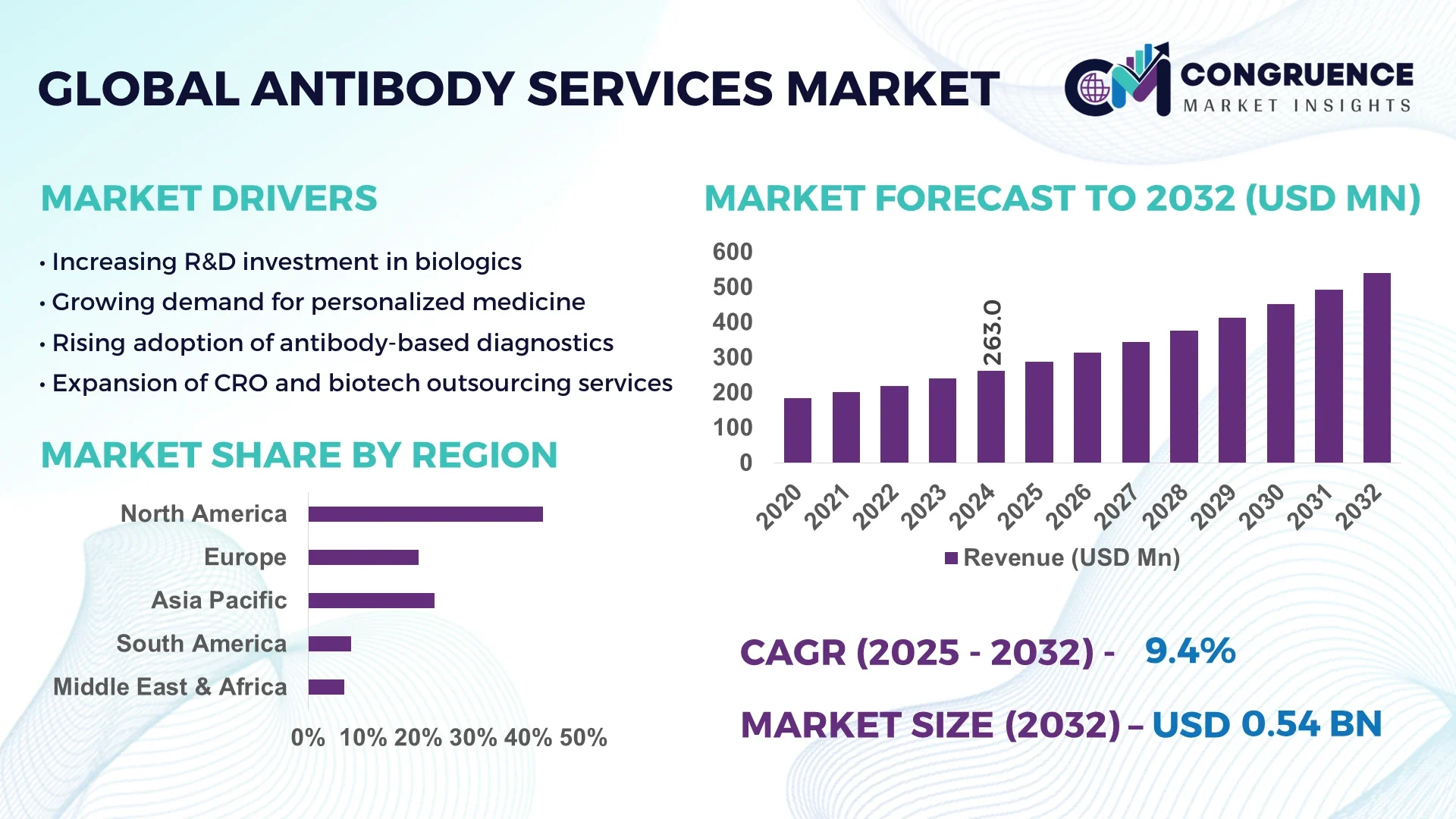
The Global Antibody Services Market was valued at USD 263.0 Million in 2024 and is anticipated to reach a value of USD 539.6 Million by 2032 expanding at a CAGR of 9.4% between 2025 and 2032.
The United States maintains a leading position in the Antibody Services Market due to its expansive biopharmaceutical R&D infrastructure, with over 1,500 dedicated antibody production facilities, continued investments surpassing USD 12 billion annually in therapeutic antibody development, and cutting-edge innovations across monoclonal and polyclonal antibody platforms for oncology and immunology applications.
The Antibody Services Market is driven by growing demand across pharmaceuticals, biotechnology, and academic research sectors, with contract research organizations (CROs) accounting for a significant portion of outsourced antibody production. Innovations such as recombinant antibody technologies, hybridoma optimization, and single-cell sequencing techniques are enabling rapid and precise antibody development. Regulatory advancements—including fast-track designations for biologics and rising biosimilar approvals—are streamlining market entry timelines, particularly in North America, Europe, and select Asia-Pacific regions. Environmentally conscious manufacturing processes and GMP-certified production protocols are further enhancing service provider competitiveness. Increasing global research budgets, especially in oncology, autoimmune diseases, and infectious diseases, continue to stimulate demand. Additionally, strategic collaborations between pharmaceutical companies and antibody service providers are driving co-development models, enabling faster preclinical-to-clinical transitions. Looking ahead, the market is witnessing a shift toward fully human antibodies, automation of antibody screening, and integration of AI to optimize selection, all of which are expected to expand service depth and enhance delivery timelines across regional hubs.
Artificial Intelligence (AI) is reshaping the Antibody Services Market by enhancing accuracy, accelerating timelines, and optimizing workflows throughout the antibody discovery and development lifecycle. AI-driven platforms are increasingly integrated into target identification, antibody sequence design, and structure prediction—minimizing the need for iterative lab testing and reducing cycle times by up to 40%. Machine learning algorithms enable predictive modeling of antibody-antigen interactions, allowing researchers to eliminate non-viable candidates early in the pipeline. Natural language processing (NLP) tools are aiding in the analysis of vast scientific literature to identify novel biomarkers and therapeutic targets. Furthermore, AI-supported data analytics platforms are used to analyze high-throughput screening results, improving selection precision and shortening lead identification phases. Digital twins and in silico testing are also helping companies simulate large-scale antibody responses, reducing in vivo testing reliance. Notably, companies adopting AI platforms are experiencing up to 30% improvement in operational performance across design and validation stages. This transformation is not only enabling cost-effective operations but also improving scalability for custom antibody services. AI is fostering a data-driven approach that streamlines collaboration between CROs, pharmaceutical companies, and academic institutions, thus redefining service delivery standards in the Antibody Services Market.
“In April 2025, a leading biotech firm integrated deep generative models into its antibody discovery platform, resulting in a 65% reduction in antibody candidate screening time and a 25% increase in binder affinity success rate across oncology-related applications.”
The Antibody Services Market is undergoing significant transformation due to evolving research priorities, advanced biomanufacturing methods, and increasing collaboration between academic and commercial sectors. Rising biologics pipelines, particularly in oncology and autoimmune diseases, are generating a consistent need for scalable antibody generation services. Enhanced automation in antibody screening and production is reducing human error and enabling faster output without compromising quality. The industry is witnessing growth in demand for monoclonal antibody services, custom antibody generation, and humanized antibodies tailored for preclinical and clinical research. Additionally, regional players are expanding their capabilities in Asia-Pacific and Europe, driven by increasing R&D investments and favorable regulatory reforms. These dynamics collectively reflect a maturing market ready for adaptive innovations and operational scaling.
One of the primary drivers of the Antibody Services Market is the rapid expansion of R&D activities in the global biopharmaceutical sector. Companies are significantly increasing investments in antibody-based drug development, especially for oncology, infectious diseases, and autoimmune conditions. In 2024 alone, over 600 biologics—including monoclonal and bispecific antibodies—were in various stages of the pipeline. The increasing focus on next-generation antibody formats, such as antibody-drug conjugates and nanobodies, is boosting the demand for specialized service providers capable of high-throughput and precision-based antibody engineering. Government funding initiatives in countries like the U.S., Germany, and China are also contributing to the robust development pipeline by supporting both academia and commercial ventures in antibody research.
Despite growing demand, a significant restraint in the Antibody Services Market is the lack of standardized protocols across service providers. Variations in quality control, immunization strategies, and purification methods lead to inconsistencies in antibody yield, specificity, and reproducibility. This becomes critical in applications involving diagnostic kit development and therapeutic screening, where consistency is vital. Additionally, IP-related complexities and unclear contractual terms between clients and service providers further complicate project execution. As a result, some biopharmaceutical companies prefer in-house antibody development to mitigate these uncertainties, reducing the market potential for outsourced service providers unless robust compliance and quality assurance frameworks are implemented.
The Antibody Services Market is witnessing a surge in opportunities due to the expanding use of antibodies in precision medicine and companion diagnostics. The shift toward individualized treatment approaches is fueling demand for highly specific monoclonal and polyclonal antibodies tailored to unique patient profiles. Diagnostic antibody services are gaining traction in early cancer detection and stratification of autoimmune disorders. Advances in bioinformatics and genomic sequencing are also enabling researchers to identify novel biomarkers, which in turn require customized antibody development for validation. This trend is pushing service providers to enhance offerings in rapid antibody generation and humanized antibody formats, thereby opening new revenue channels and accelerating market expansion.
A major challenge facing the Antibody Services Market is the complexity of manufacturing workflows and the associated regulatory compliance burdens. The production of therapeutic-grade antibodies involves intricate steps such as cell line development, upstream and downstream processing, and quality control under strict cGMP conditions. Each of these stages requires validation and documentation that align with global regulatory standards, including those set by the FDA and EMA. Delays in regulatory approvals, batch rejections, and the cost of maintaining cleanroom environments significantly impact time-to-market and profitability. Smaller service providers often struggle to keep up with evolving compliance demands, thereby limiting scalability and long-term growth prospects in the global market.
Surge in Single-Domain Antibody Applications: The demand for single-domain antibodies, also known as nanobodies, is on the rise due to their enhanced tissue penetration and stability. In 2025, multiple biotech firms initiated over 40 clinical investigations involving nanobodies, targeting neurological and respiratory conditions. Their production simplicity and superior binding affinity are influencing service providers to expand capabilities in nanobody design and validation.
Automation of Antibody Screening Platforms: Automated liquid handling systems and AI-powered analysis tools are being increasingly adopted to streamline antibody screening processes. These systems are enabling 24/7 workflows and reducing screening timelines by up to 50%. In high-throughput facilities, robotics-enabled ELISA and flow cytometry platforms have become the standard for rapid and reproducible antibody characterization.
Growth in Outsourced Research Models: Pharmaceutical companies are progressively outsourcing antibody development to specialized CROs and CDMOs. In 2024, over 60% of early-stage antibody discovery projects in North America were handled by external partners. This trend is expanding service provider portfolios and encouraging cross-border collaborations focused on therapeutic antibody development.
Rise in Modular and Prefabricated Construction: The adoption of modular construction is reshaping demand dynamics in the Antibody Services Market. Pre-bent and cut elements are prefabricated off-site using automated machines, reducing labor needs and speeding project timelines. Demand for high-precision machines is rising, especially in Europe and North America, where construction efficiency is critical.
The Antibody Services Market is broadly segmented by type, application, and end-user, each representing a distinct dimension of market demand and operational focus. These segments reflect the diversity of services provided across research, development, and clinical pipelines. In terms of type, monoclonal and polyclonal antibody services remain the most widely utilized due to their broad application in diagnostics, therapeutics, and R&D. Applications of antibody services span areas such as oncology, infectious diseases, immunology, and neurobiology, with oncology leading due to rising investments in targeted therapies. The market’s end-user landscape includes pharmaceutical and biotechnology companies, academic and research institutions, and contract research organizations (CROs). Pharmaceutical companies dominate due to their active biologics pipelines, while CROs are gaining momentum as outsourcing increases. This segmentation highlights how innovations, disease-specific demands, and the need for scalable research infrastructure are shaping consumption patterns and driving strategic alignment across global regions.
The Antibody Services Market includes several service types, notably monoclonal antibodies, polyclonal antibodies, recombinant antibodies, and custom antibody services. Among these, monoclonal antibody services are the most prominent, driven by their critical role in developing precision therapeutics and biologics. These antibodies offer high specificity, which is particularly valuable in applications such as targeted cancer therapies and chronic autoimmune conditions. The increasing demand for FDA-approved monoclonal therapies has pushed both large and mid-sized service providers to expand their production and validation capacities.
Recombinant antibody services represent the fastest-growing segment due to their enhanced scalability, batch-to-batch consistency, and reduced immunogenicity. These features are crucial in meeting the rising quality standards in therapeutic and diagnostic markets. The ability to customize recombinant antibodies for specific applications also makes them attractive for both research and clinical use.
Polyclonal antibodies continue to serve niche applications, particularly in academic research and low-cost diagnostic assay development due to their ease of production and multi-epitope recognition. Meanwhile, custom antibody services cater to specialized requirements across research and biotech startups, offering tailored solutions with rapid turnaround. Each type plays a vital role in meeting diverse demand across global antibody pipelines.
Applications of antibody services span a wide array of biomedical and pharmaceutical domains, with oncology being the dominant application segment. The widespread use of monoclonal antibodies for cancer immunotherapies and companion diagnostics has significantly influenced demand in this area. Numerous ongoing clinical trials and approvals for antibody-based oncology drugs are sustaining consistent demand for antibody discovery, engineering, and validation services.
The fastest-growing application segment is infectious diseases, driven by global efforts to develop antibody-based diagnostics and therapeutics for viruses and bacterial pathogens. The COVID-19 pandemic accelerated investments in this space, and ongoing concerns over future pandemics are keeping demand elevated. In particular, recombinant antibodies are playing a key role in developing rapid diagnostic kits and neutralizing therapies.
Other important applications include autoimmune disorders, where antibody therapies are increasingly being used to modulate immune responses, and neurological diseases, where research is focusing on targeted antibodies for conditions such as Alzheimer’s and Parkinson’s. Additionally, metabolic disorders and transplantation immunology are emerging as focus areas, reflecting the market’s broadening application base and its alignment with evolving medical priorities.
The Antibody Services Market is significantly influenced by the diversity of its end-user base. Pharmaceutical and biotechnology companies form the largest end-user segment, leveraging antibody services to support extensive biologic pipelines, accelerate drug discovery timelines, and reduce in-house development costs. These organizations rely heavily on outsourced services for high-throughput screening, humanization, and preclinical validation of therapeutic antibodies.
Contract research organizations (CROs) are the fastest-growing end-user segment, as the biopharma industry continues to embrace outsourcing models to enhance flexibility and cost efficiency. CROs benefit from scalable infrastructure and technological specialization, enabling them to serve both emerging biotech startups and large pharmaceutical companies. Their increasing participation in early-stage antibody discovery projects is transforming the landscape of service delivery.
Academic and research institutions also play a vital role in driving innovation within the market. These institutions contribute significantly to early antibody target identification and proof-of-concept studies. In recent years, public–private partnerships have enhanced collaboration between academia and industry, enabling knowledge transfer and supporting translational research. Together, these end-users form a synergistic ecosystem, each contributing to the dynamic evolution of the Antibody Services Market.
North America accounted for the largest market share at 42.6% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 10.5% between 2025 and 2032.
This regional distribution reflects distinct market maturity and innovation drivers. North America’s leadership stems from a well-established pharmaceutical ecosystem, robust government funding, and a high concentration of CROs. Meanwhile, Asia-Pacific’s momentum is fueled by expanding biotech hubs in countries like China and India, rapidly improving manufacturing infrastructure, and growing investments in translational research. Europe remains a vital contributor, driven by strong academic-industry collaboration and progressive regulatory support. South America and the Middle East & Africa are emerging markets showing increased antibody consumption across diagnostics and therapeutic domains. Collectively, global regions are shaping a geographically diverse yet interconnected antibody services landscape focused on innovation, scalability, and localization of advanced healthcare solutions.
North America held the largest share of the Antibody Services Market in 2024, accounting for approximately 42.6% of global demand. The United States continues to be the core contributor, supported by a well-established biopharmaceutical industry and significant investment in monoclonal antibody development. Canada is also emerging with increased biotech funding and academic collaborations. Key industries driving demand include oncology, autoimmune therapeutics, and precision diagnostics. Notably, the FDA’s continued support for accelerated approval pathways has encouraged rapid service expansion. Technological advancements—such as AI-powered screening and automated antibody engineering platforms—are transforming service delivery models. Digital biomanufacturing initiatives, including cloud-based lab management systems, are further optimizing performance and quality assurance across contract service providers. The region's innovation-centric approach and favorable regulatory environment make it a critical growth hub for global antibody service providers.
Europe contributes approximately 29.4% to the global Antibody Services Market, with Germany, the United Kingdom, and France leading in service adoption and innovation. The region’s strength lies in its strong academic-research networks and public-private partnerships that accelerate early-stage antibody discovery. Regulatory bodies such as the European Medicines Agency (EMA) continue to support biosimilar and biologic innovation through structured guidance and funding frameworks. Germany remains at the forefront of custom antibody manufacturing, while the UK sees rapid growth in diagnostic antibody services. Across Europe, green chemistry and sustainability initiatives are increasingly integrated into antibody production practices. Adoption of advanced technologies such as automated screening, CRISPR integration, and AI-assisted antibody modeling is expanding, positioning Europe as a progressive region with deep capabilities in therapeutic and diagnostic antibody services.
Asia-Pacific is positioned as the fastest-growing regional market in the Antibody Services Market, with countries like China, India, and Japan emerging as top contributors. In 2024, the region accounted for roughly 17.8% of global service demand, with substantial gains in custom antibody development and contract manufacturing. China's aggressive investment in bioscience parks and antibody R&D facilities is reinforcing local production capacity. India’s rising demand for diagnostic and therapeutic antibodies, supported by its expanding pharmaceutical exports, is further driving market growth. Japan continues to lead in antibody engineering and high-precision lab automation. Regional innovation hubs like Singapore and South Korea are also gaining traction. Infrastructure development, including smart laboratories and bioprocessing plants, is accelerating scalability. Asia-Pacific's evolving regulatory frameworks and increasing clinical trial activities signal long-term strategic importance in the global antibody services ecosystem.
In 2024, South America accounted for approximately 5.3% of the Antibody Services Market, with Brazil and Argentina serving as primary contributors. Brazil's growing life sciences sector and public healthcare investments have driven localized antibody production, particularly for infectious diseases and immunodiagnostics. Argentina is emerging with niche biotech startups offering regional antibody development services. Infrastructure development in pharmaceutical hubs, particularly in São Paulo and Buenos Aires, is supporting growth. Government-backed innovation grants and updated trade policies are enabling greater access to global technology partnerships. Although still developing, South America's market potential is supported by growing research ecosystems, public-private collaborations, and a clear focus on improving diagnostic coverage and therapeutic antibody accessibility.
The Middle East & Africa region accounted for around 4.9% of the global Antibody Services Market in 2024, with notable demand emerging from UAE, South Africa, and Saudi Arabia. These countries are investing in local biotech infrastructure and diagnostic research facilities to reduce dependency on imports. The rise of infectious disease testing and national health screening programs has spurred demand for regionally produced antibodies. Technological modernization—including laboratory automation, digital monitoring systems, and GMP-aligned cleanroom facilities—is being rapidly adopted in the Gulf Cooperation Council (GCC) states. Trade agreements and regulatory reforms, such as streamlined approvals for diagnostics and biologics, are fostering regional partnerships and increasing foreign direct investment in antibody service infrastructure. The MEA market is gradually evolving with a strong push for capacity building, workforce training, and innovation localization.
United States – 39.8% Market Share
High production capacity and continuous R&D investment from global pharmaceutical leaders dominate the Antibody Services Market in the United States.
China – 14.6% Market Share
Strong end-user demand, rapid infrastructure expansion, and government-led biotech programs make China a key driver in the Antibody Services Market.
The Antibody Services Market features a moderately fragmented but increasingly competitive landscape with over 100 active global and regional service providers. Leading companies maintain strong positions through comprehensive service portfolios, established client relationships, and global delivery capabilities. Mid-sized firms are gaining ground by offering customized antibody services, niche technologies, and flexible pricing models tailored to specific research or therapeutic needs. Strategic initiatives are shaping the competitive dynamics, including frequent partnerships between biotech firms and CROs, licensing collaborations, and capacity expansion in key geographic markets such as North America, Europe, and Asia-Pacific.
Mergers and acquisitions are being pursued to strengthen regional footprints and vertically integrate antibody development and production workflows. Companies are also investing in next-generation antibody platforms, such as bispecific antibodies and humanized monoclonals, to enhance differentiation. The adoption of AI and automation tools for faster target identification and antibody engineering is becoming a key innovation driver. Moreover, contract manufacturing alliances and long-term service agreements with pharmaceutical firms are increasing as outsourcing becomes more prevalent. The overall competitive focus remains on scalability, regulatory compliance, and rapid service delivery to meet growing biologics demand.
Abcam plc
GenScript Biotech Corporation
Bio-Rad Laboratories, Inc.
Thermo Fisher Scientific Inc.
Creative Biolabs
Abnova Corporation
ProteoGenix
Sino Biological Inc.
Aragen Life Sciences
Pacific Immunology Corp.
Rockland Immunochemicals, Inc.
Bioventix PLC
Technological innovation is a central pillar shaping the current and future growth of the Antibody Services Market. One of the most significant advancements includes the use of recombinant DNA technology for the development of high-fidelity monoclonal antibodies, which offer enhanced specificity and reproducibility across applications in diagnostics, therapeutics, and research. Additionally, single B-cell cloning is being adopted for rapid screening and identification of antibodies with optimal binding affinities, significantly reducing development time from months to a few weeks.
Automation technologies, such as robotic liquid handling systems and AI-driven screening platforms, are streamlining high-throughput screening and sequence optimization. Laboratories using automated ELISA and flow cytometry platforms report up to 50% improvements in processing efficiency. The integration of AI and machine learning in structure-function prediction, epitope mapping, and immunogenicity analysis is also accelerating preclinical workflows and enabling early identification of lead candidates.
Further, next-generation sequencing (NGS) and CRISPR-based screening are helping service providers identify and validate novel antibody targets, improving development accuracy and lowering the risk of clinical failure. Cloud-based data platforms and digital lab environments are enabling real-time collaboration between global teams, ensuring faster delivery cycles and regulatory compliance. The increasing use of bioinformatics pipelines and in silico modeling is driving cost-effective service customization and broadening client engagement across small biotech firms and global pharmaceutical companies alike.
• In March 2024, GenScript Biotech launched a high-throughput antibody humanization platform that reduces project turnaround time by 30% while maintaining over 90% binding affinity across a broad range of therapeutic targets.
• In August 2023, Thermo Fisher Scientific expanded its biologics facility in Massachusetts, adding 85,000 sq. ft. of antibody development capacity to serve increasing outsourcing demand from mid-sized biopharma firms.
• In December 2023, Creative Biolabs introduced an AI-enhanced antibody-antigen interaction predictor, achieving 92% prediction accuracy and reducing the need for wet lab testing by 40%.
• In May 2024, Sino Biological inaugurated a new R&D facility in Frankfurt, Germany, focusing on recombinant antibody development and production to cater to the European diagnostic and vaccine development markets.
The Antibody Services Market Report offers a comprehensive analysis of the global landscape, encompassing key segmentation across type, application, and end-user categories. The report evaluates service types such as monoclonal, polyclonal, recombinant, and custom antibodies, highlighting their specific roles in therapeutic, diagnostic, and research domains. It delves into application segments like oncology, infectious diseases, autoimmune disorders, and neuroscience, offering detailed insight into how each vertical is shaping service demand.
Geographically, the report covers North America, Europe, Asia-Pacific, South America, and the Middle East & Africa, with a special focus on country-level performance in major markets such as the United States, China, Germany, and Brazil. The analysis includes regional trends in infrastructure development, manufacturing capacity, and technology adoption.
Additionally, the report explores the role of advanced technologies including AI, automation, single-cell screening, and bioinformatics platforms, examining how these innovations are driving efficiencies and expanding service capabilities. It also highlights the growing importance of outsourcing, partnerships, and integrated solutions in the market ecosystem. The scope further extends to emerging service models, such as hybrid CRO-CDMO offerings and personalized antibody solutions, presenting a strategic view of the market’s future trajectory tailored to stakeholders in pharma, biotech, and research sectors.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2024) | USD 263.0 Million |
| Market Revenue (2032) | USD 539.6 Million |
| CAGR (2025–2032) | 9.4 % |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End‑User
|
| Key Report Deliverables | Revenue Forecast, Growth Drivers & Restraints, Technology Insights, Market Dynamics, Segmentation Analysis, Regional Insights, Competitive Landscape, Recent Developments |
| Regions Covered | North America, Europe, Asia‑Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Abcam plc, GenScript Biotech Corporation, Bio-Rad Laboratories, Inc., Thermo Fisher Scientific Inc., Creative Biolabs, Abnova Corporation, ProteoGenix, Sino Biological Inc., Aragen Life Sciences, Pacific Immunology Corp., Rockland Immunochemicals, Inc., Bioventix PLC |
| Customization & Pricing | Available on Request (10 % Customization is Free) |
