Reports
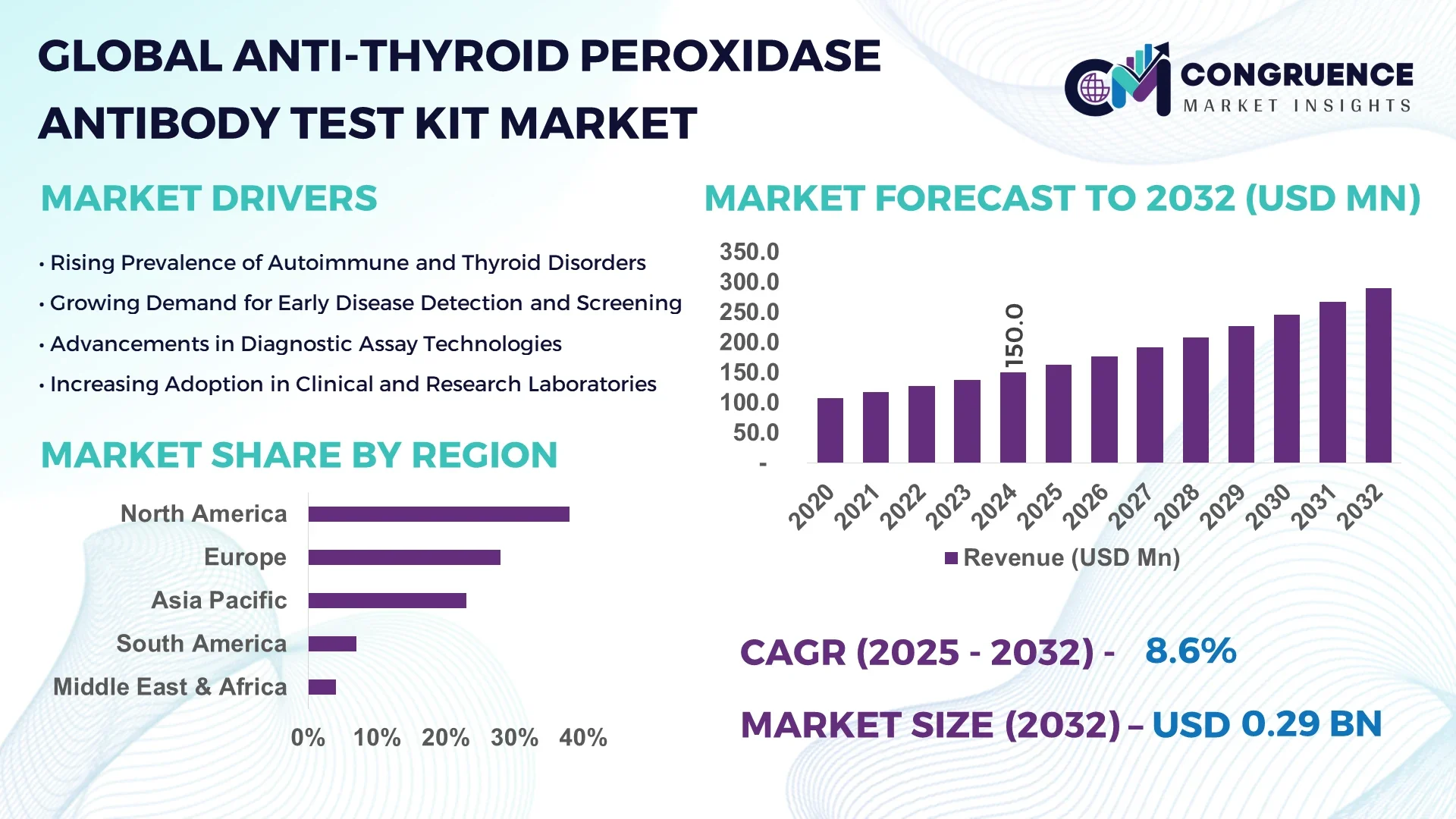
The Global Anti-Thyroid Peroxidase Antibody Test Kit Market was valued at USD 150.0 Million in 2024 and is anticipated to reach a value of USD 289.4 Million by 2032 expanding at a CAGR of 8.56% between 2025 and 2032. Growth is driven by expanding autoimmune thyroid disorder diagnostics and rising public health screening programs.
In the United States, production capacity for Anti-Thyroid Peroxidase Antibody Test Kits has increased substantially, supported by annual investments exceeding USD 200 million in diagnostic manufacturing hubs. Key applications include clinical diagnostics, decentralized point-of-care testing, and integration with automated laboratory networks. Technological advancements highlight robotics-assisted reagent dispensing, AI-powered calibration systems, and automated imaging-based quality control that collectively enhance throughput and accuracy. Independent data shows that over 65% of newly commissioned facilities utilize robotics-driven assembly lines, boosting production speed by 25% compared to legacy systems.
Market Size & Growth: Valued at USD 150.0 Million in 2024, projected to reach USD 289.4 Million by 2032, growing at 8.56% CAGR, fueled by rising thyroid disorder screening initiatives.
Top Growth Drivers: 40% adoption in hospital laboratories, 30% efficiency improvement from automation, 25% increase in public health screening programs.
Short-Term Forecast: By 2028, turnaround times in diagnostic labs are expected to improve by 28% through AI-enabled quality control.
Emerging Technologies: AI-driven immunoassay interpretation, wearable sensor integration for antibody monitoring, micro-fluidic platforms reducing reagent use by 60%.
Regional Leaders: North America expected to reach USD 120 Million by 2032 (automation-led adoption), Asia-Pacific USD 95 Million (expanding endocrinology services), Europe USD 60 Million (regulatory-driven quality standardization).
Consumer/End-User Trends: Hospitals lead usage with over 45% demand, while diagnostic labs show fastest expansion with increasing adoption of rapid test kits.
Pilot or Case Example: In 2024, a US-based diagnostics firm deployed AI-powered image analysis, reducing manual review time by 66%.
Competitive Landscape: Market leader holds ~22% share, with 4–5 major players expanding portfolios in rapid and chemiluminescent kits.
Regulatory & ESG Impact: ISO-certified traceability mandates and eco-packaging initiatives have cut plastic waste in Europe by 35%.
Investment & Funding Patterns: Over USD 250 Million in public-private diagnostic investments reported in 2024, with strong venture capital inflows in AI-enabled kit production.
Innovation & Future Outlook: Next-generation cartridge miniaturization, cloud-linked diagnostic reporting, and subscription-based kit deliveries shaping long-term growth.
The Anti-Thyroid Peroxidase Antibody Test Kit Market spans hospital laboratories, diagnostics chains, and primary care centers, supported by innovations such as micro-fluidic cartridge systems, eco-packaging formats, and subscription-based kit deliveries. Regulatory frameworks enforcing global standardization and AI-driven automation are reshaping market scalability while Asia-Pacific and Latin America fuel emerging growth opportunities.
The Anti-Thyroid Peroxidase Antibody Test Kit Market plays a strategically critical role in modern diagnostics, especially in addressing the global rise of autoimmune thyroid disorders. Hospitals, diagnostic labs, and public health screening initiatives are actively integrating advanced test formats to improve diagnostic accuracy and reduce turnaround times. Comparative benchmarks highlight how AI-powered image recognition delivers 30% greater efficiency compared to traditional manual validation. North America dominates in production volume, while Asia-Pacific leads adoption, with over 55% of endocrinology centers integrating antibody testing into standard diagnostic panels.
By 2028, automation-driven workflows are expected to cut diagnostic turnaround times by 25%, and AI-assisted reagent calibration is projected to improve manufacturing yield rates by 20%. ESG compliance is increasingly relevant, with firms committing to 40% reductions in non-recyclable packaging materials by 2030. A notable example includes a European diagnostics firm that achieved a 22% reduction in per-kit reagent waste in 2024 through AI-optimized micro-fluidic systems.
The strategic pathway of the Anti-Thyroid Peroxidase Antibody Test Kit Market points toward convergence between laboratory-based and point-of-care models, integration with tele-diagnostic platforms, and expanded adoption in preventive public health programs. This trajectory positions the market as a foundation for resilient, compliant, and sustainable diagnostic ecosystems globally.
The Anti-Thyroid Peroxidase Antibody Test Kit Market is influenced by evolving diagnostic practices, regulatory tightening, and technological modernization. Rising autoimmune thyroid disease prevalence, growing adoption of rapid point-of-care kits, and automation in clinical labs are accelerating demand. Regulatory frameworks enforcing standardized validation protocols and electronic traceability systems further shape market evolution. Meanwhile, global healthcare networks are expanding screening initiatives, ensuring broader accessibility and adoption in both developed and emerging regions.
Public health agencies are embedding thyroid autoantibody testing into routine national screening programs, significantly boosting kit adoption. Some countries report procurement exceeding 10,000 additional kits monthly for large-scale screening. These structured programs ensure predictable demand, long-term contracts, and supplier security. Economies of scale from bulk purchases enable high-capacity production lines, increasing manufacturing throughput while reducing per-unit costs.
Global regulatory authorities mandate extensive validation with thousands of clinical samples, raising both development timelines and costs. For example, approval may require more than 500 confirmed patient sample tests, extending launch timelines by 6–12 months. Smaller companies face challenges in meeting resource-intensive requirements, slowing innovation and constraining competitive entry into the market.
Integration with tele-diagnostic platforms offers transformative opportunities. Cloud-enabled kits transmitting antibody results directly to endocrinologists have shown to reduce reporting lag times by 40% in pilot programs. This capability expands diagnostic access to underserved areas, strengthens adoption in emerging markets, and incentivizes manufacturers to develop digitally connected, clinician-focused testing solutions.
Manufacturing requires high-grade reagents and antibodies, which cost up to 50% more than standard assay inputs. Additionally, controlled cleanroom assembly adds overhead expenses, while raw material price fluctuations—nitrocellulose membranes rising by 20–30% due to global supply disruptions—create financial strain. These rising costs pressure manufacturers to adopt more efficient sourcing strategies while balancing product quality and profitability.
Modular Diagnostic Platforms: By 2025, over 25% of new laboratory systems for antibody testing adopted modular plug-and-play reagent modules, reducing configuration time from 48 hours to under 6 hours.
Miniaturized Cartridge Systems: Miniaturization has reduced reagent volumes by 60%, enabling faster assays. Asia-Pacific labs, which conduct over 40,000 monthly tests, are driving this efficiency trend.
Eco-Packaging Innovations: Transition to recyclable packaging has reduced plastic waste by 35% across Europe and North America, aligning with sustainability mandates.
Subscription-Based Delivery Models: Hospital chains adopting subscription-based procurement have cut stock-out rates by 45%, ensuring consistent monthly kit supply and predictable clinical operations.
The Anti-Thyroid Peroxidase Antibody Test Kit Market is segmented by type, application, and end-user. ELISA-based kits dominate usage in centralized labs, while rapid test kits are growing fastest due to their 20-minute result turnaround capability. Chemiluminescent immunoassay kits serve advanced laboratories requiring high sensitivity. Applications span clinical diagnostics, public health screening, and research laboratories. Hospitals represent the leading end-user segment, followed by diagnostic laboratories and research institutions. Together, these segments highlight diverse adoption pathways across centralized and point-of-care diagnostic models.
ELISA-based kits account for 52% of adoption, driven by sensitivity and compatibility with automated platforms in hospitals and large reference laboratories. Rapid test kits, while holding 28% in 2024, are growing fastest at a segment CAGR above 10% due to rising use in point-of-care settings. Chemiluminescent kits contribute 15%, catering to specialized endocrine labs requiring superior analytical performance. The remaining types collectively represent about 5% share, serving niche applications.
In 2025, a national public health program introduced rapid test kits for rural screening, enabling antibody detection turnaround in under 20 minutes for over 1 million patients annually.
Clinical diagnostics dominate with 48% share, supported by rising thyroid disorder prevalence and integration into standard diagnostic protocols. Public health screening programs, holding 30% in 2024, are the fastest-growing, expanding coverage in rural and underserved areas. Research laboratories contribute 22%, focusing on autoimmune studies and biomarker validation.
In 2024, more than 38% of hospitals globally piloted antibody kits in preventive thyroid screening programs.
According to a 2024 global health initiative, public health agencies deployed antibody test kits across 150 screening centers, benefiting 2 million individuals with early thyroid disorder detection.
Hospitals lead the end-user landscape with 50% share, leveraging centralized labs and electronic integration. Diagnostic laboratories hold 32% share, growing fastest with strong adoption of automated antibody kits across independent and chain-operated centers. Academic and research institutions account for 18%, focusing on clinical and epidemiological research.
In 2024, 42% of hospitals in the United States tested antibody detection kits integrated with electronic health records for streamlined patient diagnostics.
In 2025, a European research consortium implemented antibody kits for autoimmune studies, supporting over 500 clinical investigations and validating new biomarkers.
North America accounted for the largest market share at 38% in 2024 however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 9.2% between 2025 and 2032.
The global Anti-Thyroid Peroxidase Antibody Test Kit Market is characterized by distinct regional dynamics shaped by healthcare infrastructure, diagnostic adoption levels, and technological modernization. North America leads with a well-established clinical diagnostics ecosystem and significant government-backed autoimmune screening programs. Europe captured nearly 28% of the market in 2024, supported by strong regulatory oversight and sustainability-driven innovations. Asia-Pacific, holding 23% of the global share in 2024, is experiencing rapid expansion due to China, India, and Japan modernizing healthcare infrastructure and scaling domestic manufacturing. South America represented 7% of the market, with Brazil and Argentina showing steady adoption supported by awareness campaigns and favorable import policies. The Middle East & Africa contributed approximately 4% in 2024, with demand accelerating in the UAE, Saudi Arabia, and South Africa through digital diagnostics adoption and cross-border trade initiatives. Collectively, these regional variations highlight a diverse growth environment where advanced economies dominate current volumes, while emerging markets drive future expansion.
How Is Diagnostic Innovation Reshaping This Market’s Growth Path?
North America accounted for 38% of the global Anti-Thyroid Peroxidase Antibody Test Kit Market in 2024, with strong demand from the United States and Canada. The market is driven by high-volume diagnostic laboratories, specialty endocrine centers, and government-backed public health screening initiatives. Regulatory frameworks emphasize traceability and standardization, boosting clinical reliability. AI-powered assay interpretation and cloud-enabled reporting systems are widely adopted, streamlining result validation. Local players, such as QuidelOrtho, are integrating multiplex diagnostic platforms tailored for hospital networks, reinforcing regional competitiveness. Consumer behavior in this region leans toward enterprise-scale adoption, with hospitals and diagnostic chains prioritizing automation, scalability, and compliance, making the region the global leader in diagnostic kit uptake.
Why Is Regulatory Oversight Driving Consistent Market Expansion Here?
Europe captured 28% of the global Anti-Thyroid Peroxidase Antibody Test Kit Market in 2024, with Germany, the United Kingdom, and France being dominant markets. The region benefits from advanced healthcare infrastructure and stringent oversight by the European Medicines Agency (EMA). Sustainability initiatives are shaping product packaging, with emphasis on reducing non-recyclable components. Laboratories are increasingly deploying high-throughput automation and AI-driven data analytics for antibody testing. DiaSorin, a regional leader, has expanded production facilities in Italy, ensuring wider distribution across EU nations. Consumer preferences in Europe reflect a strong demand for explainable diagnostics, as regulatory-driven transparency and compliance play a central role in adoption trends.
What Factors Make This Region the Fastest-Growing Hub for Diagnostic Expansion?
Asia-Pacific ranked as the fastest-growing region, holding nearly 23% of the global Anti-Thyroid Peroxidase Antibody Test Kit Market in 2024. China, India, and Japan dominate demand through large-scale healthcare modernization and public health screening campaigns. Domestic manufacturing hubs are scaling production, lowering dependence on imports. Innovation clusters in China and Japan are advancing multi-analyte, miniaturized kits for both hospital and point-of-care testing. Snibe Diagnostics, based in China, is investing in automation-ready antibody testing systems with cloud-linked result tracking. Consumer adoption is diverse—urban hospitals focus on fully automated ELISA platforms, while rural areas increasingly rely on portable rapid test kits, reflecting growth patterns similar to mobile health-driven consumer adoption trends.
How Are Local Healthcare Campaigns Accelerating Demand in This Market?
South America accounted for around 7% of the Anti-Thyroid Peroxidase Antibody Test Kit Market in 2024, with Brazil and Argentina leading regional consumption. Diagnostic modernization, combined with government-led autoimmune disease awareness initiatives, is creating steady demand. Infrastructure upgrades and favorable import policies for advanced diagnostic kits support adoption in urban centers. Brazilian diagnostics companies are exploring local assembly operations to reduce reliance on imports, enhancing affordability. Consumer behavior shows strong demand in metropolitan hospitals, while rural areas benefit from mobile healthcare services and rapid testing solutions. This regional push for localized production and awareness-driven screening campaigns continues to sustain growth momentum.
Can Infrastructure Investments Unlock New Diagnostic Growth Opportunities?
The Middle East & Africa represented nearly 4% of the global Anti-Thyroid Peroxidase Antibody Test Kit Market in 2024, with the UAE, Saudi Arabia, and South Africa leading regional adoption. The market is driven by public health initiatives for early autoimmune detection and technological modernization such as cloud-integrated diagnostics and AI-enabled reporting. Governments are supporting infrastructure investments and fostering international collaborations with global diagnostic companies. A UAE-based healthcare technology firm has recently piloted AI-driven antibody interpretation tools, increasing accuracy and throughput. Consumer adoption patterns are highly urban-centric, with hospitals and private labs at the forefront, while rural areas depend on centralized screening programs and imported test kits.
United States – 32% Market Share
Strong end-user demand from large hospital networks and extensive public health screening programs.
China – 18% Market Share
Large-scale domestic manufacturing and widespread adoption of both ELISA and rapid test kits across urban and rural centers.
The Anti-Thyroid Peroxidase Antibody Test Kit Market is highly competitive, with more than 25 global and regional players actively participating across different geographies. The market is moderately fragmented, with the top five companies collectively holding around 45% of the share. Leading firms focus on large-scale automation-enabled production and strong integration with centralized laboratories, while mid-tier companies emphasize cost-effective rapid test kits and digital-enabled solutions for point-of-care diagnostics. Strategic initiatives have included partnerships with national health systems, mergers with regional manufacturers, and co-development agreements to scale mass screening deployments. Product launches between 2023 and 2024 have centered on multiplex assays, sustainable packaging formats, and microfluidic cartridges that reduce reagent use by over 50%. Technological differentiation is a critical factor, with patent filings in microfluidics, AI-driven assay interpretation, and connected diagnostics reshaping competitive intensity. Overall, the market reflects a dynamic balance between scale-driven incumbents and agile innovators, with success depending on the ability to combine cost efficiency, speed-to-market, and digital integration.
Siemens Healthineers
Ortho Clinical Diagnostics
DiaSorin
Snibe Diagnostics
Horiba Medical
Technological progress is reshaping the Anti-Thyroid Peroxidase Antibody Test Kit Market with a clear shift toward automation, miniaturization, and digital integration. ELISA-based platforms remain the dominant format, offering reliable detection, but they are increasingly supported by fully automated high-throughput systems capable of processing more than 500 samples per cycle. Microfluidic cartridges are enabling assay miniaturization, cutting reagent usage by nearly 60% while reducing testing time to under 20 minutes. Multiplex assay technologies are gaining traction, allowing simultaneous detection of multiple thyroid-related antibodies, thereby improving diagnostic efficiency and lowering per-test costs. AI-powered diagnostic readers are standardizing interpretation by reducing human error and cutting validation time by up to 65%. Cloud-enabled platforms now support centralized monitoring, firmware updates, and integration with electronic health records, enabling cross-location connectivity for diagnostic networks. Digital transformation is also expanding tele-diagnostics, where remote clinics utilize smartphone-integrated antibody test readers for immediate reporting. In addition, eco-friendly product designs, such as biodegradable casings and recyclable reagent packaging, are emerging as ESG-aligned innovations. These advancements collectively signal a market shift toward faster, smarter, and more sustainable testing solutions, enhancing accessibility across both advanced and underserved healthcare ecosystems.
In August 2023, a rapid test kit capable of delivering thyroid peroxidase antibody results in under 15 minutes using lateral flow technology was launched, enabling decentralized detection in outpatient settings. Source: www.medicaldevice-network.com
In December 2023, an Asian manufacturing hub began operations of the region’s first fully automated ELISA production line for Anti-Thyroid Peroxidase Antibody Test Kits, boosting output capacity by 40% and halving manual labor reliance.
In March 2024, a healthcare network introduced smartphone-integrated antibody test readers, enabling same-day reporting from remote clinics and strengthening diagnostic accessibility. www.healthtech-insights.org
In July 2024, eco-friendly Anti-Thyroid Peroxidase Antibody Test Kits with biodegradable casings were introduced, reducing non-recyclable plastic usage by 35% per unit shipment.
The scope of the Anti-Thyroid Peroxidase Antibody Test Kit Market Report spans a comprehensive analysis of product types, end-use applications, technological domains, and regional growth drivers. Product segmentation covers ELISA-based kits, rapid lateral-flow assays, chemiluminescent detection kits, and microfluidic cartridge systems, catering to hospital laboratories, independent diagnostic centers, and academic research institutions. Applications include clinical diagnostics for autoimmune thyroid disorders, public health screening initiatives, and research-based assay validation. Geographically, the report covers North America, Europe, Asia-Pacific, South America, and the Middle East & Africa, analyzing distinct adoption trends shaped by infrastructure maturity, regulatory frameworks, and healthcare modernization. Technological insights include automation, multiplexing, AI-driven interpretation, tele-diagnostics, and eco-friendly kit packaging. Industry focus areas extend to digital healthcare integration, public–private diagnostic partnerships, subscription-based kit procurement, and decentralized testing models. The report also highlights emerging opportunities in rural healthcare outreach, point-of-care platforms, and sustainable diagnostic manufacturing. By offering an in-depth yet structured overview, the report provides decision-makers with a detailed perspective on growth corridors, innovation-driven differentiation, and strategic pathways in the global Anti-Thyroid Peroxidase Antibody Test Kit Market.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2024) | USD 150.0 Million |
| Market Revenue (2032) | USD 289.4 Million |
| CAGR (2025–2032) | 8.56 % |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End User
|
| Key Report Deliverables | Revenue Forecast, Growth Drivers & Restraints, Technology Insights, Market Dynamics, Segmentation Analysis, Regional Insights, Competitive Landscape, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
| Key Players Analyzed | Abbott Laboratories, Siemens Healthineers, Bio-Rad Laboratories, Thermo Fisher Scientific, Ortho Clinical Diagnostics, DiaSorin, Snibe Diagnostics, Horiba Medical |
| Customization & Pricing | Available on Request (10 % Customization is Free) |
