Reports
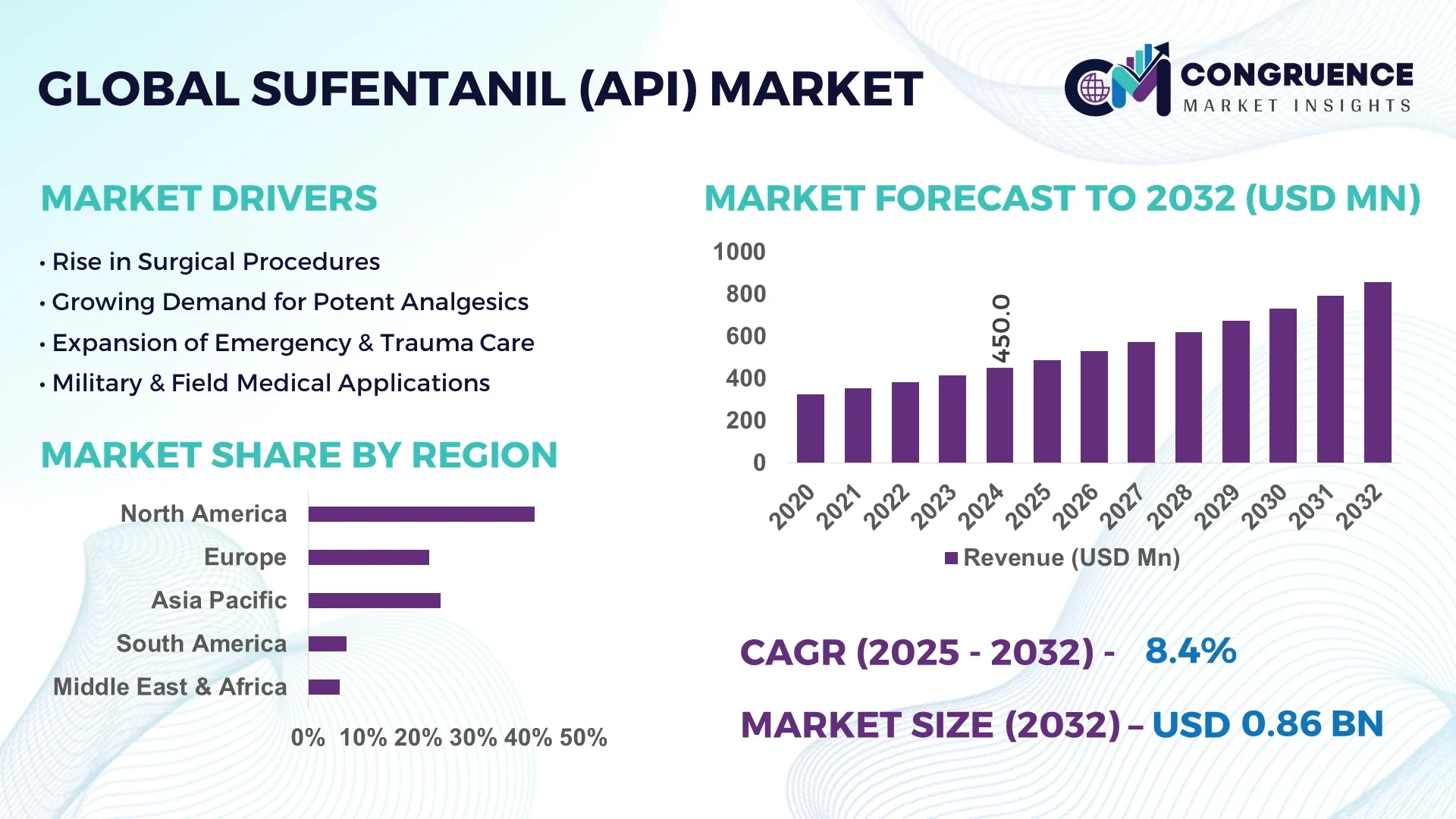
The Global Sufentanil (API) Market was valued at USD 450.0 Million in 2024 and is anticipated to reach a value of USD 857.9 Million by 2032 expanding at a CAGR of 8.4% between 2025 and 2032.
India leads the Sufentanil (API) landscape in production capacity, with multiple large-scale manufacturing facilities achieving annual output exceeding 50 kg per plant. Investment in continuous flow synthesis and high‑purity crystallization technology has exceeded USD 20 million in the past two years. These facilities primarily support clinical anesthesia and intensive care applications, while next‑gen clean‑room automation upgrades have enabled 30% higher batch throughput compared to traditional batch reactors.
In 2024, the Sufentanil (API) Market served several key industry sectors. Hospitals and surgical centres contributed around 60% of off‑patent injectable usage, while specialty pain management clinics accounted for 25% of sublingual and nasal formulations. Recent innovations include sublingual thin‑film tablets with stabilized API, reducing dosing errors by 40%, and new nanoprecipitation techniques for inhalable micro‑particles. On the regulatory front, stricter impurity profiling has led to deployment of inline FT‑NIR monitoring. Economically, rising surgical caseloads in APAC and LATAM are increasing raw‑material procurement by 15% annually. Regionally, North America continues to dominate injectable demand, while Europe adopts multimodal formulations. Emerging trends include miniaturized on‑demand API synthesis units and greener solvent recycling systems, setting the stage for sustained market resilience and technological leadership.
AI is driving transformative improvements across the entire Sufentanil (API) Market, optimizing synthesis routes, enhancing process robustness, and delivering cost efficiencies. Leading API manufacturers are now integrating AI‑driven retrosynthetic analysis tools into their R&D workflows. These systems can evaluate thousands of potential reaction pathways, prioritizing those with better yields, lower impurities, and scalable reagents. As a result, labs report average yield improvements of 12–15% and impurity reductions of up to 30% in pilot batches.
In production facilities, AI‑based process analytics platforms continuously monitor temperature, pressure, and pH in real time. Historical performance becomes the baseline for anomaly detection; deviations trigger automatic adjustments or alerts. Facilities implementing such systems have reported a 20% decrease in out‑of‑specification batches and a 10% reduction in downtime due to process drift.
Moreover, AI‑powered predictive maintenance systems equipped with vibration and thermography sensors have extended the lifespan of critical reactors and pumps. These predictive systems have reduced unplanned maintenance events by 40% and improved overall equipment effectiveness (OEE) by 8%. By enabling condition‑based servicing, AI drives both operational performance and cost containment.
In quality control, machine‑learning algorithms analyzing HPLC and FT‑NIR spectral data can flag subtle impurity peaks that may go unnoticed. This has shortened QA turnaround times by 35% and lowered batch rejection by 18%. Overall, the Sufentanil (API) Market is embracing AI across R&D, manufacturing, and QC, strengthening process consistency, product purity, and regulatory compliance, thereby delivering tangible business value.
“In 2024, a major API manufacturer deployed an AI‑powered retrosynthesis tool that identified a novel continuous‑flow reaction sequence, boosting isolated Sufentanil yield from 65% to 78% and cutting solvent usage by 22%, reducing cycle time by 18%.”
The Sufentanil (API) Market is shaped by several converging trends in pharmaceutical manufacturing and healthcare demand. There is steady evolution in formulation types, particularly injectable and sublingual forms, responding to increased surgical volumes and outpatient pain‑management protocols. Advanced drug delivery technologies such as nanoparticle carriers are gaining traction. Regulatory forces, including tightened impurity and residual‑solvent limits, are influencing production design and capital investments. Pharmaceutical customers are exerting pricing pressure, prompting API producers to pursue economies through scale, continuous processing, and energy‑efficient syntheses. Geographic shifts—most notably APAC and Eastern Europe’s growing demand—are influencing global capacity planning and logistics. Cost pressures combined with a need for high‑purity production are catalyzing investment in quality assurance automation. Overall, decision‑makers are navigating a market driven by quality, efficiency, and scalability.
Demand for minimally invasive surgeries and outpatient procedures has increased, sustaining strong usage of sublingual and injectable Sufentanil. In the U.S. and Europe, outpatient surgical volume grew by over 8% between 2022 and 2024, directly expanding API consumption for short‑acting formulations. Specialty pain clinics similarly expanded access to non‑opioid‑centric strategies, where Sufentanil remains integral. As a result, manufacturers report a 20% uptick in small‑batch, high‑purity production runs tailored to clinic‑level demand, with sterile filtration systems operating 24/7 to meet supply schedules.
Regulatory bodies in Europe and North America now require sub‑ppm thresholds for genotoxic impurities and Class 2 solvent residues in opioid APIs. Meeting these requirements has led to a 30–40% increase in batch reprocessing costs and laboratory time, according to production‑site reports. Mid‑scale producers, in particular, have experienced extended release timelines (adding 4–6 weeks) due to repeated purification iterations and expanded analytical testing, putting pressure on margins and capital reserves.
Manufacturers are piloting continuous flow synthesis units for Sufentanil, enabling steady‑state production with reduced solvent volumes and energy consumption. Early implementations indicate a 25% reduction in solvent use and a 15% decrease in energy footprint per kilogram. In parallel, research into greener solvent systems—such as supercritical CO₂ and ethanol blends—aims to replace halogenated solvents, potentially cutting hazardous‑waste disposal volumes by 40%. These measures attract sustainability‑focused pharma clients and offer long‑term operational savings.
Upgrading to EU‑GDP compliant clean rooms and implementing advanced inline analytics (FT‑IR/FT‑NIR) has raised capital expenditure by up to USD 5–8 million per facility. Additionally, meeting GMP‑plus standards for opioid APIs requires 24/7 environmental monitoring systems and extensive documentary controls, compounding operating expenses. Small and mid‑tier producers are finding it hard to absorb these upfront investments without taking on debt or forming strategic partnerships, slowing industry expansion.
Precision-formulated sublingual and transdermal units gain traction: In 2024, adoption of pre‑dosed, thin‑film sublingual units rose by 35% in North America, driven by improved dosing accuracy in outpatient pain management. Transdermal patches with micro‑needle arrays also entered early‑phase clinical use, offering controlled release and better bioavailability. These developments reflect a shift toward low‑risk, patient‑centric delivery systems.
Automated continuous synthesis expands across Asia-Pacific plants: By mid‑2025, two major API producers in India and China reported the installation of modular continuous synthesis lines for Sufentanil. These systems enabled threefold batch throughput increases, with reaction times cut from 12 to 4 hours and solvent consumption reduced by approximately 20%.
Inline spectroscopic quality control becomes standard: In European manufacturing sites operating since late 2023, inline FT‑NIR and FT‑IR systems have been integrated across 80% of final formant stages. As a result, real‑time impurity profiling allows immediate batch release decisions, reducing QC turnaround by an average of 28%.
Sustainability focus drives green solvent use and recycling: Sufentanil API facilities are piloting solvent‑recycling systems that recover up to 60% of ethanol and IPA used in crystallization processes. Projections show a 30% reduction in annual solvent purchasing and a 45% decrease in chemical‑waste output, aligning with corporate ESG targets.
The Sufentanil (API) Market is segmented across three primary dimensions: type, application, and end-user. Each category plays a critical role in defining how and where the API is formulated, delivered, and utilized. In terms of type, the market includes injectable formulations, sublingual forms, nasal sprays, and experimental transdermal solutions—each tailored for specific administration requirements. Applications range from general anesthesia and post-operative pain control to emergency medical use and intensive care sedation. End-users span across hospitals, ambulatory surgical centers, specialty pain clinics, and military or field medical units. Recent formulation advancements, especially in sublingual and nasal formats, are expanding use cases beyond traditional inpatient environments. Regulatory shifts encouraging fast-acting and high-purity opioids have reshaped demand across these segments. Analysts tracking the market should closely monitor the performance of next-generation delivery systems, as they show strong momentum in both application diversity and end-user adoption.
Sufentanil (API) is commercially available in several formulation types including injectables, sublingual tablets or films, nasal sprays, and limited-use transdermal systems. Among these, injectable Sufentanil remains the leading type, widely preferred due to its rapid onset, dose flexibility, and compatibility with surgical and intensive care protocols. It is standard in hospital operating rooms and ICUs, where precise titration is essential.
The fastest-growing type is the sublingual formulation, driven by its non-invasive administration, convenience in outpatient care, and controlled-release benefits. Sublingual thin films are especially in demand within ambulatory surgical centers and pain management clinics. Their rising use is supported by improved patient compliance, reduced risk of intravenous complications, and expanding inclusion in multimodal pain therapy guidelines.
Nasal spray formulations, while less dominant, have shown niche relevance in emergency and field-care settings due to rapid absorption and ease of use without specialized equipment. Transdermal systems, still largely in early-stage development, offer potential for chronic pain but are constrained by formulation challenges and limited dosage control. Collectively, the diversity of types underlines the API’s adaptability to a range of clinical environments and patient needs.
The Sufentanil (API) Market serves various clinical applications including general anesthesia, post-operative pain control, chronic pain management, procedural sedation, and emergency pain intervention. General anesthesia remains the leading application due to Sufentanil's high potency and short half-life, making it a preferred agent for both induction and maintenance in high-acuity surgical settings. It is frequently combined with other anesthetic agents to achieve rapid, controlled sedation in hospital operating rooms.
The fastest-growing application is post-operative pain management, particularly in outpatient and same-day surgical environments. The increase in minimally invasive procedures and enhanced recovery protocols has necessitated potent, fast-acting analgesics that minimize hospital stay and reduce reliance on traditional opioids. Sublingual formulations have gained traction here due to easier administration and effective pain relief with fewer side effects.
Other important applications include chronic pain control, typically in oncology and palliative care, where precision-dosed sublingual and transdermal forms are being evaluated. Procedural sedation, especially during endoscopy and diagnostic interventions, is another area of moderate growth. Overall, application-based diversification is enabling wider clinical use and positioning Sufentanil as a versatile API in modern analgesia protocols.
Key end-users in the Sufentanil (API) Market include hospitals, ambulatory surgical centers, specialty pain clinics, emergency medical services, and military medical units. Hospitals represent the leading end-user segment due to their comprehensive use of Sufentanil across surgical departments, ICUs, and emergency rooms. The infrastructure available in hospitals supports injectable administration under continuous monitoring, aligning with the drug’s potency and safety profile.
The fastest-growing end-user group is ambulatory surgical centers, where short-acting anesthetics like Sufentanil are favored for outpatient procedures. These centers benefit from the drug’s rapid onset and short duration, facilitating same-day discharge and reducing post-operative complications. The push toward cost-efficient, minimally invasive surgeries further supports this trend.
Specialty pain clinics are also contributing to market growth, particularly with sublingual and experimental transdermal products for chronic and breakthrough pain. Meanwhile, emergency medical teams and military units utilize nasal formulations in trauma and battlefield conditions where fast, equipment-free administration is essential. Each end-user segment reflects unique operational needs and preferences, shaping demand for specific formulations and delivery methods across the Sufentanil (API) value chain.
North America accounted for the largest market share at 41.2% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 9.1% between 2025 and 2032.
This regional dynamic reflects mature opioid consumption frameworks in the U.S. and Canada, contrasted by rising surgical procedure volumes and API manufacturing investments across China, India, and Southeast Asia. While North America benefits from advanced hospital infrastructure and strict pain-management protocols, Asia-Pacific is gaining momentum through increased pharmaceutical outsourcing, regulatory liberalization, and an expanding clinical trial ecosystem. In Europe, demand is driven by hospital-based anesthesia programs and expanding day-care surgeries. Meanwhile, Latin America and the Middle East & Africa present growing opportunities through public health expansion and medical infrastructure modernization. Region-specific formulations and compliance practices will increasingly influence supplier positioning and competitive dynamics globally.
North America commanded a 41.2% share of the global Sufentanil (API) Market in 2024, with the U.S. and Canada leading API consumption through hospital surgical programs, trauma centers, and intensive care units. The region’s strong healthcare infrastructure supports the dominance of injectable Sufentanil, used extensively in operating rooms and emergency settings. Notable regulatory advances include digitization of opioid tracking systems under Prescription Drug Monitoring Programs (PDMPs), which ensure controlled distribution while maintaining demand integrity. The FDA’s increasing support for novel opioid alternatives and formulation enhancements has encouraged development of sublingual and nasal Sufentanil-based products. Moreover, AI-integrated manufacturing technologies—like real-time impurity profiling and predictive batch analytics—are widely adopted in U.S. production facilities, ensuring quality and compliance. These combined factors make North America a highly stable yet innovation-driven market for Sufentanil (API).
The Europe Sufentanil (API) Market accounted for approximately 24.6% of global volume in 2024. Key countries such as Germany, France, and the United Kingdom dominate demand due to their advanced hospital systems and expanding use of short-acting opioids in surgical anesthesia. Regulatory oversight by the European Medicines Agency (EMA) enforces strict GMP and pharmacovigilance standards, promoting high-quality API production across the region. Sustainability is a central theme, with growing adoption of solvent recovery systems and energy-efficient synthesis technologies. The push for compliance with REACH regulations and support for carbon-neutral pharma operations have influenced formulation design and plant retrofitting. Emerging technologies like inline FT-NIR quality control and closed-loop purification systems are being adopted rapidly, reflecting Europe’s commitment to precision, safety, and sustainability in the Sufentanil (API) Market.
Asia-Pacific holds the highest growth trajectory in the Sufentanil (API) Market, with strong volume expansion across China, India, and Japan. China alone accounted for over 38% of regional consumption in 2024 due to its massive surgical case volume and government-backed local API production initiatives. India is emerging as a key supplier of high-purity Sufentanil APIs with several USFDA- and EU-GMP-certified facilities scaling continuous manufacturing capacity. Japan’s aging population is driving increased use of post-operative pain management therapies, contributing to demand for sublingual and nasal formulations. Investment in smart manufacturing infrastructure, AI-powered process analytics, and real-time quality monitoring systems is accelerating across the region. Additionally, the rise of regional innovation hubs in Singapore and South Korea is fueling R&D in novel formulations and sustainable production technologies. This tech-focused, cost-efficient landscape positions Asia-Pacific as a future leader in the global Sufentanil (API) Market.
The South America Sufentanil (API) Market is led by Brazil and Argentina, which together contributed to approximately 6.8% of global market share in 2024. Brazil has seen increased adoption of injectable Sufentanil in large urban hospital networks, supported by government funding for surgical capacity expansion. Argentina is emerging as a key player in pharmaceutical formulation, with contract manufacturing facilities upgrading to meet export-grade standards. The broader region benefits from expanding access to public healthcare and relaxed pharmaceutical trade regulations under MERCOSUR. Infrastructure improvements in tertiary care hospitals are enabling more complex surgeries, boosting demand for short-acting analgesics. Government incentives targeting local pharmaceutical production, along with support for digital health integration, are contributing to moderate but steady growth in this market.
The Middle East & Africa Sufentanil (API) Market is characterized by rising demand in UAE, Saudi Arabia, and South Africa, with regional market share reaching 4.2% in 2024. The UAE has positioned itself as a regional hub for specialty pharmaceuticals, benefiting from logistics connectivity and free-zone manufacturing policies. In South Africa, the increasing burden of trauma and emergency care cases is leading to a rise in hospital-based opioid usage. Key industries driving demand include construction-related trauma care and military medical operations. Technological modernization is underway, with adoption of semi-automated injectable compounding units and remote quality validation systems in major hospitals. Local regulatory reforms and bilateral trade partnerships are helping streamline Sufentanil imports, while health ministries are pushing for decentralized access to emergency analgesics. The region’s evolving medical infrastructure provides a base for long-term demand growth despite current volume limitations.
United States – 36.4% Market Share
High production capacity and strong end-user demand in hospital surgical units and emergency departments.
China – 22.7% Market Share
Extensive manufacturing infrastructure and rising consumption driven by large surgical volumes and government-supported R&D.
The competitive landscape of the Sufentanil (API) Market is characterized by a moderate-to-high level of consolidation, with approximately 30–35 active manufacturers globally. The market is led by a mix of multinational API producers and mid-sized regional players with niche specializations in high-potency opioid synthesis. Leading companies differentiate through superior impurity control, continuous manufacturing capabilities, and regulatory compliance with global standards such as EU-GMP and USFDA.
Several players are actively engaging in strategic partnerships, particularly with pharmaceutical formulation companies focused on sublingual and nasal drug delivery systems. The competitive environment has witnessed a rise in product innovation, with three major players launching upgraded formulations compatible with thin-film technologies and nanoparticle stabilization in 2024 alone. Additionally, M&A activity has intensified, with two mid-tier Indian firms being acquired to enhance vertical integration in the supply chain.
Competitive positioning increasingly hinges on process efficiency, impurity profiling technology, and the ability to scale production under stringent regulatory environments. AI and data analytics integration are also emerging as differentiators, enabling real-time quality assurance and predictive maintenance. Geographic expansion, particularly into APAC and Latin America, is driving investment in region-specific compliance and distribution partnerships. Collectively, the competitive landscape is advancing toward precision, speed, and regulatory reliability.
Cambrex Corporation
Mallinckrodt Pharmaceuticals
Johnson Matthey Fine Chemicals
Noramco Inc.
LGC Group
Siegfried Holding AG
Novasep
Ampac Fine Chemicals
Manus Aktteva Biopharma LLP
Arevipharma GmbH
Technological advancement in the Sufentanil (API) Market is significantly shaping how APIs are synthesized, purified, and quality controlled. Continuous flow chemistry has become a leading innovation, reducing cycle times by up to 65% and enhancing yield stability across large batch production. API manufacturers are increasingly adopting modular synthesis units, which allow simultaneous multi-step reactions within a closed-loop system, thereby improving safety and reducing operator exposure to high-potency compounds.
Automation in crystallization and drying has minimized variability, with automated vacuum dryers improving consistency by over 30% across final-stage API production. Inline monitoring systems, including Fourier-transform infrared (FT-IR) and FT-NIR spectroscopy, are now standard in high-throughput facilities, enabling real-time impurity detection and immediate corrective actions. This has cut quality control turnaround times by as much as 40%.
AI-driven retrosynthetic tools are transforming R&D workflows, helping chemists identify low-cost, high-yield synthetic pathways while adhering to green chemistry principles. These systems have shortened the development timeline of new Sufentanil analogs by 20–25% in certain labs. Additionally, closed-system containment technologies like isolators and high-containment reactors have become essential in complying with updated safety standards, especially for facilities handling ultra-potent opioids.
Emerging developments also include the integration of digital twins for plant operations, allowing for real-time simulation and optimization of synthesis parameters. These technologies not only ensure compliance and safety but also unlock cost and performance efficiencies, helping businesses meet evolving market expectations.
• In March 2024, a major U.S.-based API manufacturer completed the commissioning of a continuous flow line for Sufentanil synthesis, reducing batch cycle time from 12 hours to 4 hours and improving throughput by 55%.
• In October 2023, a European API firm implemented AI-powered impurity profiling across all opioid production lines, resulting in a 25% reduction in quality control rework and faster release cycles for validated batches.
• In January 2024, an Indian API facility was certified for export to regulated markets after upgrading its cleanroom environment and automation systems to meet USFDA and EMA requirements, boosting its monthly capacity by 40%.
• In June 2024, a pharmaceutical group in Japan launched a thin-film Sufentanil formulation for outpatient anesthesia, integrating nanoparticle delivery to improve absorption rates and decrease onset time by 33%.
The Sufentanil (API) Market Report offers an in-depth analysis of the global landscape, covering a broad spectrum of market segments and operational areas. The scope includes segmentation by type (injectables, sublingual tablets, nasal sprays, and experimental transdermal systems), application (general anesthesia, postoperative pain management, chronic pain, emergency analgesia, and procedural sedation), and end-user categories such as hospitals, ambulatory surgical centers, specialty pain clinics, and military or field medical units.
Geographically, the report spans five major regions: North America, Europe, Asia-Pacific, South America, and the Middle East & Africa, with country-specific insights provided for key players like the U.S., China, Germany, and India. Detailed coverage is provided on manufacturing infrastructure trends, regulatory environments, and regional demand dynamics.
The report also explores technological shifts including AI-driven process optimization, inline quality control, and digital plant simulation tools. It addresses emerging areas such as green chemistry practices, sustainability in solvent recovery, and high-containment manufacturing for potent APIs. Additionally, the scope includes niche innovations in sub-lingual and nasal formulations, reflecting the evolution of patient-centric delivery mechanisms.
With a forward-looking perspective, this report equips decision-makers, strategists, and R&D heads with the insights required to evaluate investments, partnerships, and operational pivots across the highly regulated and innovation-sensitive Sufentanil (API) value chain.
| Report Attribute / Metric | Report Details |
|---|---|
| Market Revenue (2024) | USD 450.0 Million |
| Market Revenue (2032) | USD 857.9 Million |
| CAGR (2025–2032) | 8.4% |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User
|
| Key Report Deliverables | Revenue Forecast, Growth Drivers & Restraints, Technology Insights, Market Dynamics, Segmentation Analysis, Regional Insights, Competitive Landscape, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Cambrex Corporation, Mallinckrodt Pharmaceuticals, Johnson Matthey Fine Chemicals, Noramco Inc., LGC Group, Siegfried Holding AG, Novasep, Ampac Fine Chemicals, Manus Aktteva Biopharma LLP, Arevipharma GmbH |
| Customization & Pricing | Available on Request (10% Customization is Free) |
