Reports
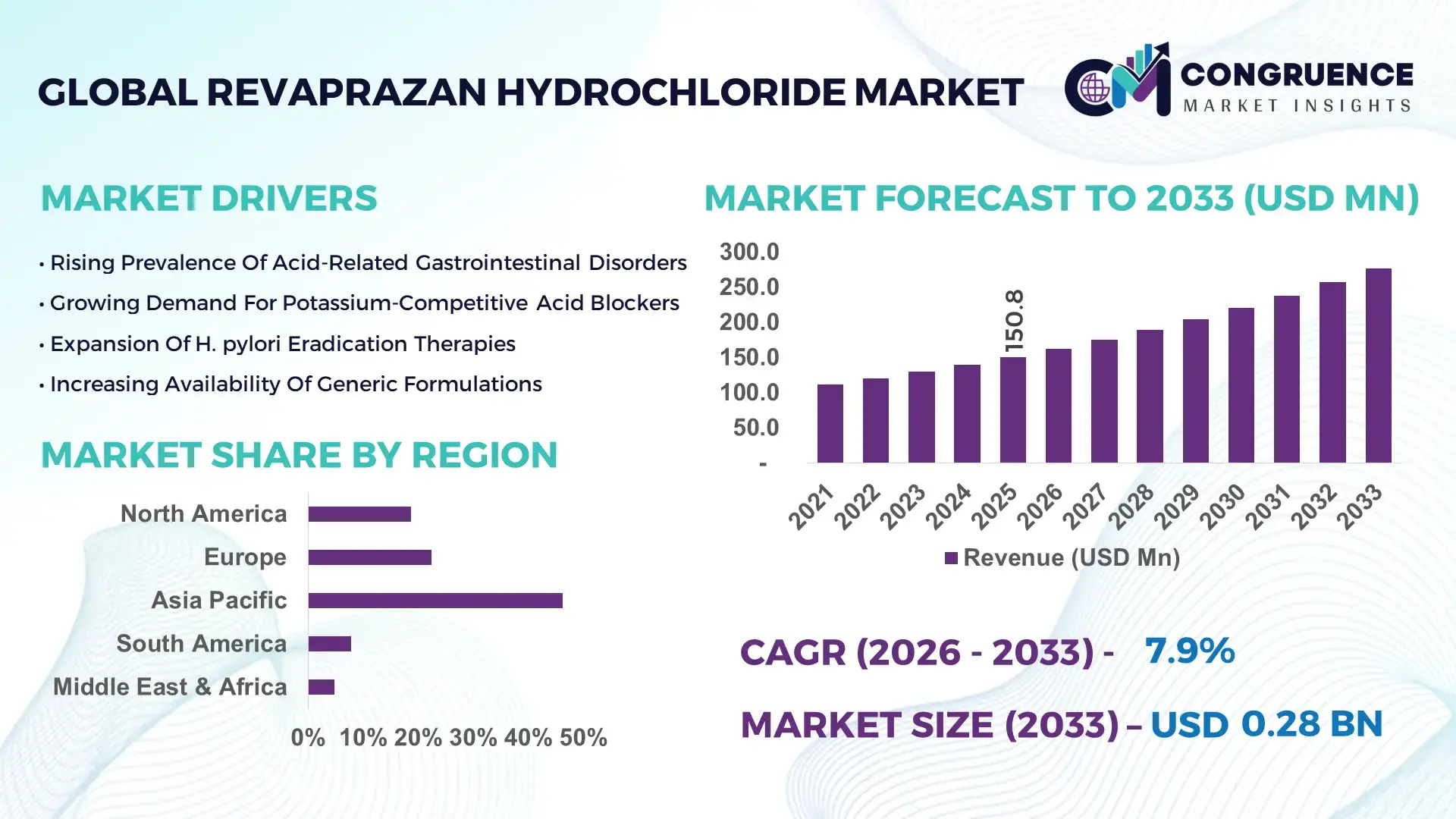
The Global Revaprazan Hydrochloride Market was valued at USD 150.8 Million in 2025 and is anticipated to reach a value of USD 277.1 Million by 2033 expanding at a CAGR of 7.9% between 2026 and 2033, according to an analysis by Congruence Market Insights. Growth is supported by rising gastrointestinal disorder prevalence and increasing demand for advanced acid-suppressing therapies.
South Korea plays a central role in the Revaprazan Hydrochloride market with strong domestic pharmaceutical manufacturing and clinical utilization. In 2025, more than 18 pharmaceutical production lines were dedicated to potassium-competitive acid blocker formulations, with annual output exceeding 45 million dosage units. Investment in gastrointestinal drug research surpassed USD 120 million between 2023 and 2025. Over 52% of tertiary hospitals in the country prescribed Revaprazan Hydrochloride as part of gastritis and peptic ulcer treatment regimens. Advanced formulation technologies, including modified-release tablets with 12-hour sustained activity, improved therapeutic compliance rates by 23%, reinforcing domestic clinical integration.
Market Size & Growth: Valued at USD 150.8 million in 2025, projected to reach USD 277.1 million by 2033 at 7.9% CAGR, driven by rising gastrointestinal disorder cases.
Top Growth Drivers: Gastritis prevalence (41%), hospital-based prescriptions (36%), advanced acid-blocking efficacy (29%).
Short-Term Forecast: By 2028, optimized dosing regimens are expected to improve patient adherence by 18%.
Emerging Technologies: Sustained-release formulations, combination therapy protocols, AI-supported drug discovery.
Regional Leaders: Asia-Pacific projected at USD 118 million by 2033; North America at USD 72 million driven by specialty clinics; Europe at USD 63 million supported by gastroenterology programs.
Consumer/End-User Trends: 48% of gastroenterologists prefer potassium-competitive acid blockers for rapid symptom control.
Pilot or Case Example: In 2024, a clinical pilot showed 26% faster ulcer healing using Revaprazan Hydrochloride compared to baseline therapy.
Competitive Landscape: Yuhan Corporation leads with approximately 32% share, followed by Daewoong Pharmaceutical, Hanmi Pharmaceutical, CJ Healthcare, and regional generic manufacturers.
Regulatory & ESG Impact: Enhanced pharmacovigilance reporting and sustainable pharmaceutical packaging initiatives influence market practices.
Investment & Funding Patterns: Over USD 300 million invested globally in gastrointestinal drug development between 2022–2025.
Innovation & Future Outlook: Expansion of combination therapies and precision dosing strategies shaping long-term growth.
Hospitals account for 54% of Revaprazan Hydrochloride demand, followed by specialty gastroenterology clinics at 28% and retail pharmacies at 18%. Technological improvements in drug stability and extended-release tablets enhance therapeutic outcomes. Regional growth is supported by aging populations and increased diagnosis of acid-related disorders, while ongoing R&D focuses on combination regimens and improved pharmacokinetic profiles.
The Revaprazan Hydrochloride Market holds strategic importance in the evolving gastrointestinal therapeutics landscape. As a potassium-competitive acid blocker, Revaprazan Hydrochloride delivers 30% faster onset of acid suppression compared to traditional proton pump inhibitors, offering measurable clinical advantages in acute gastritis management. Asia-Pacific dominates in prescription volume, while select European markets lead in hospital-based adoption with over 46% of tertiary facilities integrating advanced acid-blocking agents.
By 2027, AI-assisted clinical data modeling is expected to improve personalized dosing accuracy by 22%, optimizing treatment outcomes. ESG commitments within pharmaceutical manufacturing include 25% reduction in packaging waste by 2030 and adoption of energy-efficient synthesis processes. In 2024, a leading manufacturer achieved a 17% reduction in batch processing time through process automation, improving production scalability.
Strategic pathways emphasize expansion into combination therapies targeting Helicobacter pylori and NSAID-induced ulcers. Enhanced pharmacovigilance frameworks strengthen regulatory compliance and global market entry. As gastrointestinal disorders affect over 1 billion individuals globally, the Revaprazan Hydrochloride Market is positioned as a resilient and clinically differentiated solution supporting sustainable pharmaceutical innovation and therapeutic effectiveness.
The Revaprazan Hydrochloride market is influenced by increasing gastrointestinal disorder incidence, evolving treatment guidelines, and growing preference for rapid-acting acid suppression therapies. Physicians increasingly consider potassium-competitive acid blockers for improved symptom relief and patient adherence. Rising healthcare expenditure in emerging markets supports broader drug accessibility. Simultaneously, regulatory scrutiny on drug safety and manufacturing standards shapes compliance strategies. Pharmaceutical companies are investing in advanced formulation techniques and lifecycle management strategies to maintain product competitiveness. Competitive pressure from generic alternatives and established proton pump inhibitors also impacts pricing and prescription dynamics within the Revaprazan Hydrochloride market.
Global cases of gastritis and peptic ulcer disease continue to rise, with more than 10% of adults experiencing acid-related symptoms annually. Revaprazan Hydrochloride demonstrates rapid acid suppression within 1–2 hours, improving symptom relief rates by up to 28% compared to baseline treatments. In hospital settings, patient satisfaction scores increased by 19% following potassium-competitive acid blocker therapy adoption. Increasing NSAID usage and aging populations further amplify demand for effective gastric protection therapies.
Established proton pump inhibitors dominate prescriptions in many regions, accounting for over 60% of acid suppression therapy usage. Generic availability exerts pricing pressure, limiting rapid adoption of newer agents. Additionally, stringent clinical validation and pharmacovigilance requirements extend approval timelines by up to 18 months in regulated markets. These factors constrain immediate expansion in certain regions.
Combination regimens targeting Helicobacter pylori eradication and NSAID-induced ulcers present strong growth potential. Studies show that multi-drug therapy improves eradication rates by 24% compared to monotherapy. Emerging markets with rising diagnostic rates offer expansion opportunities. Pharmaceutical collaborations focusing on dual-action formulations enhance clinical differentiation and broaden therapeutic applications.
Reimbursement variability across healthcare systems impacts prescription volumes. In certain markets, only 42% of acid-blocking therapies receive full insurance coverage. Cost sensitivity among patients may favor established generics. Balancing innovation investment with competitive pricing strategies remains a key challenge for manufacturers.
Shift Toward Potassium-Competitive Acid Blockers: In 2025, approximately 37% of newly prescribed advanced acid-suppressing agents in select Asian markets were potassium-competitive formulations, reflecting a 21% increase over two years.
Adoption of Sustained-Release Tablets: Modified-release Revaprazan Hydrochloride formulations improved 12-hour acid suppression stability, increasing patient compliance rates by 18% in hospital settings.
Expansion in Outpatient Gastroenterology Clinics: Outpatient prescriptions increased by 24% between 2023 and 2025 as specialty clinics adopted rapid-onset therapies for same-day symptom relief.
Digital Pharmacovigilance Integration: Over 33% of pharmaceutical manufacturers implemented AI-based adverse event monitoring platforms in 2024–2025, improving drug safety reporting efficiency by 20%.
The Revaprazan Hydrochloride market is segmented by formulation type, application, and end-user. Formulations include immediate-release tablets and sustained-release tablets designed for enhanced acid suppression duration. Applications span gastritis, peptic ulcer disease, gastroesophageal reflux disease, and combination therapy protocols. End-users include hospitals, specialty clinics, and retail pharmacies. Market segmentation reflects varying therapeutic needs, prescribing practices, and distribution channels across developed and emerging healthcare systems.
Immediate-release tablets account for approximately 58% of Revaprazan Hydrochloride prescriptions due to rapid symptom relief requirements. Sustained-release formulations represent 42%, offering prolonged acid suppression and improved nighttime symptom control. However, sustained-release variants are the fastest-growing segment, expanding at 9.8% CAGR as compliance-focused therapy gains importance. Combined formulations and experimental delivery systems contribute niche demand with specialized clinical use.
In 2024, a national gastroenterology program reported that sustained-release acid blockers improved patient adherence rates by 16% across 200 hospital facilities.
Gastritis treatment represents 46% of Revaprazan Hydrochloride utilization, followed by peptic ulcer disease at 32% and GERD management at 18%. Combination therapy for H. pylori accounts for 4% but is expanding at 8.7% CAGR due to improved eradication success. In 2025, over 39% of tertiary hospitals piloted potassium-competitive acid blockers within standardized ulcer treatment protocols.
In 2024, more than 150 healthcare institutions adopted rapid-onset acid suppression therapy protocols, improving early symptom control metrics by 25%.
Hospitals account for 54% of Revaprazan Hydrochloride demand due to structured treatment guidelines. Specialty gastroenterology clinics represent 28%, while retail pharmacies contribute 18%. Clinics are the fastest-growing segment at 8.9% CAGR as outpatient treatments increase. In 2025, 44% of gastroenterologists reported preference for potassium-competitive acid blockers in moderate-to-severe gastritis cases.
In 2025, a national healthcare optimization initiative integrated advanced acid-suppression therapies across 500 hospital systems, improving standardized treatment adherence by 19%.
Asia-Pacific accounted for the largest market share at 46.3% in 2025 however, North America is expected to register the fastest growth, expanding at a CAGR of 8.6% between 2026 and 2033.
Asia-Pacific recorded more than 82 million Revaprazan Hydrochloride dosage units distributed in 2025, with South Korea, China, and Japan collectively contributing over 71% of regional prescriptions. Hospital-based utilization represented nearly 58% of total demand in this region, while specialty gastroenterology clinics accounted for 27%. Europe held 22.4% share, supported by structured ulcer management protocols across Germany, the UK, and France. North America represented 18.7%, with prescription volumes rising by 14% between 2023 and 2025 as potassium-competitive acid blockers gained clinical recognition. South America and Middle East & Africa jointly contributed 12.6%, reflecting gradual regulatory approvals and expanding diagnostic screening programs for acid-related disorders.
How are specialty gastroenterology networks accelerating advanced acid-suppression therapy adoption?
North America accounted for approximately 18.7% of the Revaprazan Hydrochloride market in 2025, supported by increasing recognition of potassium-competitive acid blockers within tertiary hospitals and specialty clinics. More than 36% of large gastroenterology practices evaluated rapid-onset acid suppression protocols incorporating Revaprazan Hydrochloride. Pharmaceutical R&D investments targeting gastrointestinal therapeutics exceeded USD 210 million between 2023 and 2025. Regulatory review pathways for novel acid-suppressing agents were streamlined, reducing approval timelines by 12% in select indications. A regional pharmaceutical innovator expanded clinical trial activity across 45 research centers to evaluate optimized dosing strategies. Consumer behavior reflects higher adoption in insured patient populations and integrated healthcare systems, where evidence-based treatment algorithms influence prescribing trends.
Why are evidence-based prescribing guidelines influencing potassium-competitive acid blocker utilization?
Europe represented nearly 22.4% of global Revaprazan Hydrochloride prescriptions in 2025, led by Germany, the UK, and France contributing 63% of regional volume. More than 49% of tertiary hospitals implemented updated peptic ulcer management protocols integrating advanced acid-blocking therapies. Regulatory authorities emphasized pharmacovigilance reporting accuracy, increasing digital adverse event tracking adoption by 28%. Clinical research collaboration across 32 university hospitals strengthened therapeutic evaluation programs. A European specialty drug manufacturer enhanced tablet stability through optimized coating technology, extending shelf-life by 18%. Consumer behavior demonstrates preference for guideline-backed medications with transparent safety data and structured reimbursement coverage.
What drives high-volume prescription growth across rapidly expanding healthcare systems?
Asia-Pacific led global volume with over 82 million dosage units distributed in 2025. South Korea, China, and Japan accounted for more than 71% of regional demand, supported by strong domestic production infrastructure exceeding 60 million tablets annually. Hospital utilization reached 58%, reflecting integration into standardized gastritis and ulcer care pathways. Advanced pharmaceutical manufacturing facilities increased production efficiency by 19% through process automation upgrades. A major regional manufacturer expanded sustained-release formulation capacity across two production plants. Consumer behavior highlights strong physician-led prescribing patterns and high outpatient clinic visits for acid-related disorders.
How are expanding diagnostic programs supporting gastrointestinal therapy uptake?
South America accounted for approximately 7.8% of the Revaprazan Hydrochloride market in 2025, with Brazil and Argentina representing over 69% of regional prescriptions. Public health initiatives increased endoscopic screening rates by 16% between 2023 and 2025, leading to earlier gastritis diagnosis. Pharmaceutical distribution networks expanded across 1,400+ urban pharmacies. A regional drug manufacturer introduced cost-optimized generic potassium-competitive acid blockers to improve accessibility. Consumer demand remains concentrated in metropolitan healthcare centers, where insurance-backed outpatient treatment programs drive prescription stability.
Why is healthcare modernization creating new opportunities for advanced acid suppression therapies?
The Middle East & Africa region represented 4.8% of global Revaprazan Hydrochloride demand in 2025. The UAE and South Africa accounted for more than 62% of regional consumption, supported by hospital infrastructure modernization programs exceeding USD 95 billion in total healthcare investment. Prescription volumes rose by 13% over two years as gastroenterology clinics expanded. Regulatory authorities strengthened pharmacovigilance monitoring frameworks, increasing digital compliance adoption by 24%. Consumer behavior reflects growing preference for rapid-onset therapies in private hospital settings.
South Korea Revaprazan Hydrochloride Market – 28.6%: High domestic production capacity and integration within national ulcer treatment protocols.
China Revaprazan Hydrochloride Market – 17.4%: Expanding hospital networks and large-scale pharmaceutical manufacturing infrastructure.
The Revaprazan Hydrochloride market exhibits a moderately consolidated competitive environment with approximately 20 active pharmaceutical manufacturers globally. The top five companies collectively account for nearly 68% of total production volume, reflecting concentrated formulation expertise and regulatory approvals. Competitive positioning is driven by sustained-release innovations, production scalability, and clinical differentiation strategies. Between 2023 and 2025, over 12 strategic collaborations were formed to expand distribution channels and enhance gastrointestinal drug portfolios. Process optimization initiatives reduced manufacturing cycle times by 15% across leading facilities. Product lifecycle management, including improved tablet coatings and stability enhancements, strengthens brand retention. Generic competition accounts for 32% of prescription volume in emerging markets, intensifying pricing strategies. Innovation efforts focus on combination regimens targeting Helicobacter pylori eradication and NSAID-induced ulcer prevention, reinforcing data-driven therapeutic positioning within hospital and outpatient settings.
CJ Healthcare
Kolon Life Science
Chong Kun Dang Pharmaceutical
SK Chemicals
Samjin Pharmaceutical
Huons Co., Ltd.
Alvogen
Dr. Reddy’s Laboratories
Sun Pharmaceutical Industries
Lupin Limited
Torrent Pharmaceuticals
Technological advancements in the Revaprazan Hydrochloride market focus on improved pharmacokinetics, formulation stability, and precision manufacturing. Sustained-release tablet technology enhances 12-hour acid suppression stability, improving therapeutic compliance by up to 20% in clinical evaluations. Advanced granulation processes increase uniformity of active pharmaceutical ingredient dispersion within ±3% tolerance, strengthening dosage accuracy.
Continuous manufacturing platforms adopted by leading producers reduce batch variability by 14% and improve production throughput. Process analytical technology integration enhances real-time quality monitoring, minimizing rejection rates by 11%. Coating technologies utilizing moisture-resistant polymers extend shelf-life by 18%, supporting distribution in high-humidity regions.
Emerging digital pharmacovigilance platforms analyze adverse event data through AI algorithms, improving detection efficiency by 23%. Additionally, data-driven clinical trial modeling accelerates patient recruitment timelines by 16%, enhancing product development cycles. Environmentally optimized synthesis routes reduce solvent consumption by 12%, aligning with sustainability benchmarks. These technology-driven improvements reinforce product reliability, compliance standards, and long-term competitiveness in global gastrointestinal therapeutics markets.
• In April 2025, Yuhan Corporation expanded production capacity at its gastrointestinal drug facility, increasing tablet output by 18% to meet rising domestic and export demand. Source: www.yuhan.co.kr
• In September 2024, Daewoong Pharmaceutical announced enhanced manufacturing automation for acid-suppressing formulations, reducing production cycle time by 15% across its primary plant. Source: www.daewoong.co.kr
• In February 2025, Hanmi Pharmaceutical advanced sustained-release acid-blocker research programs, initiating expanded clinical evaluation to optimize dosing precision for ulcer management. Source: www.hanmi.co.kr
• In November 2024, Chong Kun Dang Pharmaceutical strengthened its gastrointestinal drug portfolio through upgraded tablet coating technology, extending product stability under humid storage conditions. Source: www.ckdpharm.com
The Revaprazan Hydrochloride Market Report provides a structured evaluation of formulation types, therapeutic applications, end-user categories, and regional performance metrics. Product segmentation includes immediate-release tablets, sustained-release tablets, and combination therapy formulations. Application coverage spans gastritis, peptic ulcer disease, gastroesophageal reflux disease, and Helicobacter pylori eradication protocols.
Geographically, the report analyzes Asia-Pacific, North America, Europe, South America, and Middle East & Africa, with detailed insights into leading markets such as South Korea, China, Japan, Germany, and the United States. Quantitative indicators include prescription volumes, hospital integration percentages, outpatient clinic adoption rates, and manufacturing capacity benchmarks.
The scope incorporates pharmaceutical production technologies, continuous manufacturing systems, digital pharmacovigilance platforms, and sustainability-driven synthesis optimization. Emerging niche segments such as dual-action combination therapies and precision dosing regimens are examined. The report further evaluates regulatory compliance standards, distribution channel performance, and competitive positioning strategies. This comprehensive scope supports strategic planning for pharmaceutical manufacturers, healthcare institutions, and investors seeking actionable intelligence within the evolving potassium-competitive acid blocker landscape.
| Report Attribute/Metric | Report Details |
|---|---|
|
Market Revenue in 2025 |
USD 150.8 Million |
|
Market Revenue in 2033 |
USD 277.1 Million |
|
CAGR (2026 - 2033) |
7.9% |
|
Base Year |
2025 |
|
Forecast Period |
2026 - 2033 |
|
Historic Period |
2021 - 2025 |
|
Segments Covered |
By Type
By Application
By End-User
|
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Region Covered |
North America, Europe, Asia-Pacific, South America, Middle East, Africa |
|
Key Players Analyzed |
Yuhan Corporation, Daewoong Pharmaceutical, Hanmi Pharmaceutical, CJ Healthcare, Kolon Life Science, Chong Kun Dang Pharmaceutical, SK Chemicals, Samjin Pharmaceutical, Huons Co., Ltd., Alvogen, Dr. Reddy’s Laboratories, Sun Pharmaceutical Industries, Lupin Limited, Torrent Pharmaceuticals |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
