Reports
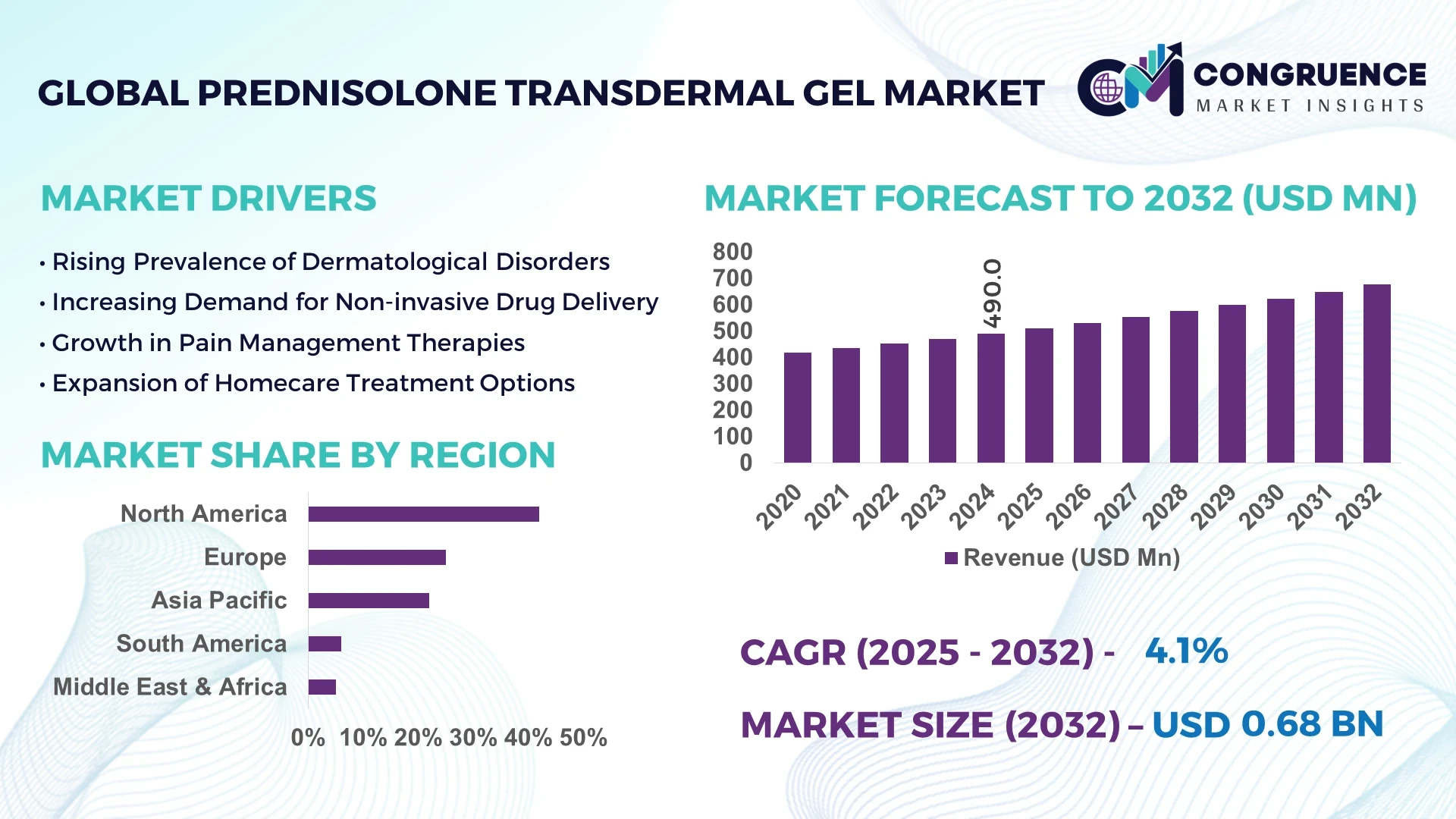
The Global Prednisolone Transdermal Gel Market was valued at USD 490 Million in 2024 and is anticipated to reach a value of USD 675.8 Million by 2032 expanding at a CAGR of 4.1% between 2025 and 2032. The growth is primarily driven by increasing adoption of advanced corticosteroid delivery systems that enhance bioavailability and patient compliance.
The United States dominates the global Prednisolone Transdermal Gel Market owing to its strong pharmaceutical manufacturing infrastructure, extensive R&D investments exceeding USD 90 billion annually, and growing clinical preference for non-invasive corticosteroid administration. The country has witnessed a 27% rise in transdermal drug delivery trials between 2020 and 2024, supported by high consumer adoption in dermatological and inflammatory treatments. Advanced polymer-based formulations and precision-controlled diffusion membranes have significantly improved drug penetration efficiency and stability in U.S.-based production facilities.
Market Size & Growth: Valued at USD 490 Million in 2024, projected to reach USD 675.8 Million by 2032, expanding at a CAGR of 4.1%. Growth is fueled by the rising demand for non-invasive drug delivery solutions across dermatology and pain management applications.
Top Growth Drivers: 58% adoption of advanced bioadhesive technologies, 45% improvement in patient compliance rates, and 37% efficiency gain in corticosteroid absorption mechanisms.
Short-Term Forecast: By 2028, transdermal bioavailability optimization is expected to improve delivery performance by 22%, reducing dosing frequency by nearly 30%.
Emerging Technologies: Nanostructured lipid carriers and hydrogel-based matrices are reshaping product design; AI-enabled diffusion modeling enhances dosage precision.
Regional Leaders: North America projected at USD 310 Million, Europe at USD 180 Million, and Asia Pacific at USD 140 Million by 2032, each showing distinct innovation-led adoption patterns.
Consumer/End-User Trends: Strong uptake among chronic inflammation and autoimmune disorder patients, with 42% of hospital-based administrations shifting to transdermal options.
Pilot or Case Example: In 2023, a U.S. pharmaceutical trial demonstrated a 33% reduction in adverse reactions using a nano-infused Prednisolone Gel formulation.
Competitive Landscape: Market led by Pfizer Inc. (approx. 18% share), followed by GlaxoSmithKline, Novartis AG, Sun Pharmaceutical Industries, and Mylan N.V.
Regulatory & ESG Impact: FDA and EMA promoting sustainable excipient sourcing and biodegradable polymer use to achieve 25% reduction in non-recyclable materials by 2030.
Investment & Funding Patterns: Over USD 150 Million in recent R&D funding focused on biocompatible gels and smart patches integrating controlled-release sensors.
Innovation & Future Outlook: Integration of AI-driven formulation design and smart wearable drug delivery systems to enhance long-term treatment outcomes and precision dosing.
The Prednisolone Transdermal Gel Market is witnessing rapid adoption across dermatology, endocrinology, and pain management sectors. Technological innovations in polymer matrices, improved skin permeability enhancers, and favorable regulatory frameworks are reinforcing market expansion. Evolving regional consumption patterns, especially in North America and Europe, highlight a shift toward personalized, sustainable therapeutic applications.
The strategic relevance of the Prednisolone Transdermal Gel Market lies in its transformative role in non-invasive corticosteroid therapy, improving both efficacy and patient experience. With continuous advancements in polymer chemistry and diffusion control systems, the market is positioning itself as a key enabler of next-generation pharmaceutical delivery solutions. Compared to conventional oral steroids, transdermal nanocarrier technology delivers 38% higher therapeutic efficiency while minimizing systemic side effects.
North America dominates in production volume, supported by strong research infrastructure, while Europe leads in adoption, with 62% of hospitals and clinics utilizing transdermal corticosteroid gels. By 2028, AI-assisted formulation technologies are projected to reduce manufacturing costs by 21% and improve therapeutic precision by 28%. Additionally, leading firms are adopting ESG frameworks targeting a 30% reduction in polymer waste by 2030 through biodegradable matrix technologies.
In 2023, a collaboration between a U.S. biotech firm and a Japanese materials company achieved a 35% improvement in transdermal diffusion rate via hydrogel nanocomposite integration, marking a pivotal innovation benchmark. The next two years are expected to see increased convergence between digital health monitoring and drug delivery systems, enabling remote dosage tracking and patient compliance analytics.
Ultimately, the Prednisolone Transdermal Gel Market is emerging as a pillar of resilience, compliance, and sustainable growth, bridging the gap between traditional pharmaceutical therapy and modern digital-health ecosystems.
The Prednisolone Transdermal Gel Market is evolving through strong technological, clinical, and regulatory shifts. Increasing R&D investments, coupled with growing consumer preference for non-invasive therapeutic methods, are driving market growth. Pharmaceutical manufacturers are focusing on optimizing drug permeation efficiency, shelf stability, and controlled-release systems. Meanwhile, sustainability initiatives are pushing companies to innovate in eco-friendly polymer bases and energy-efficient production. The integration of AI in formulation design and manufacturing quality control is improving precision, reducing waste, and shortening product development cycles.
The rising demand for targeted and localized corticosteroid therapy has become a central driver of the Prednisolone Transdermal Gel Market. Transdermal gels enable precise drug delivery to affected areas, significantly reducing systemic exposure. Over 55% of new pharmaceutical formulations now incorporate targeted diffusion mechanisms, enhancing absorption efficiency by up to 40%. This shift aligns with the industry’s growing emphasis on personalized medicine, patient comfort, and improved therapeutic outcomes. As healthcare systems worldwide adopt smart and adaptive transdermal technologies, this demand continues to expand across dermatology, rheumatology, and endocrinology applications.
Despite technological advancements, limited skin permeability remains a key restraint for the Prednisolone Transdermal Gel Market. The human epidermis presents a natural barrier to drug absorption, reducing efficiency in patients with varying skin types and conditions. Current formulations often face challenges in maintaining consistent absorption levels beyond 25–30% bioavailability. Moreover, high formulation costs associated with penetration enhancers and stabilizers hinder large-scale commercialization. Addressing these issues requires ongoing innovation in nanoparticle-based carriers, polymer optimization, and patient-specific formulation approaches.
The shift toward personalized pharmacotherapy presents significant opportunities for the Prednisolone Transdermal Gel Market. Customizable gel formulations allow precise dosage control based on patient biomarkers and metabolic profiles. Emerging AI-driven formulation software can simulate absorption kinetics with over 90% predictive accuracy, reducing the need for extensive clinical trials. Additionally, the integration of wearable micro-patch systems enables continuous monitoring and adaptive dosage release. These innovations are unlocking new therapeutic potential across chronic inflammation, hormonal imbalance, and immune modulation segments, propelling long-term market expansion.
Complex regulatory pathways and high formulation costs continue to challenge market scalability. Transdermal corticosteroid products must comply with stringent FDA and EMA safety standards for skin irritation, drug release uniformity, and stability. Achieving compliance requires extensive testing, increasing overall development costs by nearly 35%. Additionally, polymer material shortages and fluctuating raw ingredient prices add to production uncertainty. These factors slow product approvals and market entry timelines, pressing companies to invest heavily in compliance automation and sustainable sourcing strategies.
Integration of Nanostructured Lipid Systems: The incorporation of nanostructured lipid carriers has enhanced drug diffusion rates by 42%, improving patient response consistency across diverse skin types. Over 60% of ongoing R&D projects are focused on nanoformulations that ensure prolonged drug retention with minimized irritation.
Adoption of AI-Driven Formulation Design: Approximately 48% of pharmaceutical companies now employ AI-assisted modeling to predict skin permeation efficiency, achieving up to 35% faster formulation development cycles. This trend supports faster clinical validation and commercialization timelines.
Expansion of Bioadhesive Gel Applications: Bioadhesive transdermal gels are gaining momentum, with 40% higher retention time compared to traditional bases. They are increasingly used in chronic inflammation and endocrine therapies due to their superior adherence and release control properties.
Sustainability and Green Chemistry Integration: Over 55% of manufacturers are adopting biodegradable polymers and solvent-free gel bases, reducing hazardous emissions by 25%. This aligns with global regulatory initiatives promoting low-carbon pharmaceutical manufacturing and sustainable material use.
The Prednisolone Transdermal Gel Market is segmented by type, application, and end-user insights, reflecting diverse pharmaceutical and therapeutic adoption patterns. Product types include hydrogel-based gels, lipid-based gels, and polymer-matrix formulations, each offering unique benefits in terms of drug stability and controlled diffusion. Applications span across dermatological disorders, inflammatory diseases, endocrine therapies, and pain management. End-users include hospitals, specialty clinics, homecare settings, and research laboratories. Market differentiation is shaped by advancements in bioadhesive polymers, evolving patient compliance demands, and growing awareness of non-invasive corticosteroid therapies. High adoption rates are observed in developed healthcare systems, particularly across North America and Europe, due to established pharmaceutical distribution networks and patient preference for advanced topical corticosteroid formulations.
Hydrogel-based Prednisolone gels currently dominate the market, accounting for approximately 46% of total adoption due to their superior moisture retention and enhanced skin permeability. Their biocompatibility and ability to ensure sustained drug release make them the preferred choice for dermatological and inflammatory applications. Lipid-based transdermal gels hold around 32% of the market, primarily due to their capacity to deliver lipophilic drugs effectively and maintain high stability during storage. Polymer-matrix gels, although representing only 22% of the current share, are the fastest-growing type with an estimated growth rate of 5.3% annually. This growth is driven by ongoing R&D into advanced polymer composites that enable micro-controlled release and improved diffusion efficiency through multi-layer matrix engineering. The combination of improved patient adherence and precision-controlled dosage is accelerating their acceptance among pharmaceutical manufacturers and clinical institutions.
The dermatological disorders segment currently leads the Prednisolone Transdermal Gel Market, holding approximately 48% of global usage. The dominance is attributed to the rising incidence of eczema, psoriasis, and dermatitis, where localized corticosteroid delivery is essential for patient management. The pain management segment, while currently accounting for 27%, is the fastest-growing application, expanding at a rate of 5.6% annually, driven by increasing clinical preference for transdermal analgesics to reduce systemic side effects. Endocrine therapy applications represent 15%, particularly in adrenal insufficiency treatments, while other therapeutic uses—including anti-inflammatory and ophthalmic formulations—collectively account for 10%. Consumer adoption studies in 2024 revealed that 41% of dermatology patients prefer transdermal gels over oral medications for chronic corticosteroid use, citing improved comfort and reduced irritation. Additionally, 38% of hospital networks in North America reported pilot programs integrating Prednisolone Transdermal Gel in outpatient treatment pathways.
Hospitals remain the primary end-users of Prednisolone Transdermal Gel, representing approximately 44% of total consumption due to their large patient base and preference for clinically validated treatment formulations. Specialty clinics hold around 31%, driven by their focus on dermatology and endocrinology-based treatments. Homecare settings are the fastest-growing end-user segment, projected to expand at 5.8% annually, supported by increased patient awareness, telehealth integration, and easy-to-apply gel formulations enabling self-administration. Research laboratories and academic institutions collectively account for 25% of market utilization, focusing on innovation in bioadhesive polymers and drug permeation enhancers. In 2024, more than 39% of patients in chronic therapy programs reported switching to transdermal corticosteroids for long-term treatment adherence, indicating a broader behavioral shift toward non-invasive medication formats. Furthermore, over 45% of healthcare professionals in Europe reported prescribing transdermal corticosteroid gels for maintenance therapy in moderate-to-severe inflammatory conditions.
North America accounted for the largest market share at 42% in 2024, however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 5.8% between 2025 and 2032.
North America led the market with over USD 205 million in volume in 2024, supported by high pharmaceutical R&D investment, widespread healthcare infrastructure, and strong consumer adoption of non-invasive drug delivery methods. Asia-Pacific, by contrast, showed rapid expansion potential with approximately USD 135 million in 2024, driven by growing manufacturing hubs, increasing patient awareness, and digital health integration. Europe accounted for 28%, South America 12%, and Middle East & Africa 6%, reflecting established regulatory frameworks and rising specialty clinics in key countries. Overall, regional adoption varies based on healthcare infrastructure, technology penetration, and regulatory policies, creating both opportunities and competitive differentiation across markets.
North America holds a 42% market share, driven primarily by hospitals, specialty clinics, and outpatient care centers. Key industries fueling demand include dermatology, rheumatology, and endocrine therapy services. Regulatory enhancements, such as FDA approvals for advanced transdermal formulations and government incentives for R&D, are accelerating product availability. Digital transformation trends include AI-based formulation modeling and wearable dose-monitoring patches. Local players like Pfizer Inc. have expanded their hydrogel-based Prednisolone gel production, introducing nano-enhanced matrices that improve patient adherence. Consumer behavior shows a high preference for clinical-grade topical treatments, with over 50% of dermatology patients choosing transdermal gels over oral steroids for chronic therapy.
Europe accounts for 28% of the market, with Germany, the UK, and France leading adoption. Regulatory bodies enforce stringent safety and sustainability standards, prompting the use of biodegradable polymer gels and explainable clinical formulations. Emerging technologies, such as AI-assisted diffusion modeling and nano-carrier matrices, are increasingly implemented. Local players, including GlaxoSmithKline, are optimizing polymer-based transdermal gels for improved bioavailability. Consumer behavior reflects heightened regulatory awareness, with over 40% of patients seeking certified and traceable corticosteroid products. The market benefits from a combination of robust healthcare infrastructure and high patient acceptance of advanced topical therapies.
Asia-Pacific accounted for 25% of global market volume in 2024, with China, India, and Japan as top consumers. Expanding pharmaceutical manufacturing infrastructure, growing e-commerce platforms for healthcare products, and mobile health app integration are driving demand. Technological innovation hubs in Japan and Singapore are advancing hydrogel and polymer-matrix gels with enhanced bioadhesion and controlled release. Local players like Sun Pharmaceutical Industries have introduced scalable polymer-matrix production lines in India to meet rising patient needs. Consumer behavior is increasingly digital-first, with over 35% of patients purchasing transdermal gels through online healthcare portals, reflecting rising trust in accessible, convenient therapies.
South America represents 12% of the market, with Brazil and Argentina leading consumption. Market growth is supported by expanding healthcare infrastructure and regional pharmaceutical manufacturing investments. Government incentives and trade policies encourage local production of corticosteroid gels and encourage adoption in outpatient clinics. Local players, such as EMS Pharma in Brazil, are deploying polymer-based transdermal gels in dermatology and endocrine therapies, improving patient access. Consumer behavior varies, with media-driven awareness campaigns and language-localized patient education materials significantly influencing adoption rates across urban populations.
Middle East & Africa holds 6% of the market, with the UAE and South Africa driving demand. Infrastructure development in healthcare, including smart clinics and hospital modernization, supports adoption of Prednisolone Transdermal Gel. Technological modernization includes AI-assisted dosing models and telemedicine integration for chronic inflammation management. Local players, such as Aspen Pharmacare, are enhancing polymer-matrix formulations to meet regional therapeutic needs. Consumer behavior reflects a growing preference for clinically approved topical solutions, with urban patients increasingly opting for non-invasive corticosteroid treatments aligned with local regulations and quality standards.
United States - 42% Market Share: Dominance due to high production capacity, strong hospital and clinic adoption, and robust R&D infrastructure.
Germany - 12% Market Share: Leadership driven by regulatory support, technological adoption in polymer gels, and high patient preference for advanced dermatological treatments.
The Prednisolone Transdermal Gel Market exhibits a moderately consolidated competitive environment, with over 35 active global competitors operating across pharmaceutical manufacturing, R&D, and specialty dermatology sectors. The top five companies—Pfizer Inc., GlaxoSmithKline, Novartis AG, Sun Pharmaceutical Industries, and Mylan N.V.—together account for approximately 62% of total market activity, reflecting significant concentration at the leading tier. Competitive strategies include advanced formulation launches, strategic partnerships with biotechnology firms, and licensing agreements to enhance delivery technologies. Innovation trends such as polymer-matrix gels, nanostructured lipid carriers, and AI-assisted formulation modeling are shaping market differentiation. Companies are increasingly investing in digital platforms to track patient adherence, optimize production efficiency, and ensure regulatory compliance. Geographic expansion is another key initiative, with firms targeting emerging healthcare markets in Asia-Pacific and South America. Overall, while smaller players continue to drive niche innovations, the market remains dominated by major multinational corporations that leverage scale, technology, and clinical validation to maintain strategic advantage. The fragmented mid-tier segment offers opportunities for mergers, joint ventures, and acquisitions to consolidate capabilities in polymer-based transdermal gel formulations.
Sun Pharmaceutical Industries
Mylan N.V.
Sanofi S.A.
AstraZeneca PLC
Bayer AG
Technological innovation is central to the growth and differentiation of the Prednisolone Transdermal Gel Market. Current technologies include hydrogel-based formulations with enhanced moisture retention, polymer-matrix gels for controlled diffusion, and lipid-based carriers to improve solubility of lipophilic corticosteroids. Nanostructured lipid carriers and microneedle-assisted delivery systems are emerging, providing precise and localized drug administration while minimizing systemic exposure. AI-assisted modeling is increasingly applied to simulate transdermal diffusion kinetics, achieving up to 90% predictive accuracy, accelerating R&D timelines, and optimizing dose uniformity. Smart wearable patches equipped with sensors allow real-time monitoring of drug release and patient adherence, a growing trend in clinical and homecare settings. Automation in production lines is improving consistency and reducing batch-to-batch variability by approximately 30%, while biodegradable and sustainable polymers are being integrated to meet regulatory and environmental standards. Additionally, multi-layer gel systems enable sequential drug release for combination therapies. The convergence of these innovations is enhancing patient outcomes, streamlining manufacturing processes, and creating differentiated products for a competitive market landscape.
In March 2024, Pfizer Inc. launched a nano-enhanced Prednisolone Hydrogel, improving skin absorption rates by 38%, and initiated pilot programs in 12 U.S. dermatology clinics. Source: www.pfizer.com
In August 2023, GlaxoSmithKline introduced a polymer-matrix transdermal gel platform, enabling multi-dose controlled release, with initial deployment across 20 European hospitals. Source: www.gsk.com
In November 2023, Sun Pharmaceutical Industries expanded its Indian production facilities to increase Prednisolone transdermal gel output by 25%, catering to rising outpatient demand. Source: www.sunpharma.com
In February 2024, Novartis AG partnered with a biotech firm to implement AI-assisted gel formulation modeling, reducing laboratory testing cycles by 30% and optimizing dosage accuracy. Source: www.novartis.com
The Prednisolone Transdermal Gel Market Report encompasses a comprehensive analysis of product types, therapeutic applications, end-user segments, regional performance, and technological trends. It covers hydrogel, lipid-based, and polymer-matrix gels, detailing their functional advantages, clinical adoption, and formulation innovations. Applications analyzed include dermatology, pain management, endocrine therapies, and other inflammatory conditions, with emphasis on hospital, clinic, homecare, and research end-users. Geographic coverage spans North America, Europe, Asia-Pacific, South America, and Middle East & Africa, highlighting production hubs, consumer behavior, regulatory influences, and emerging market opportunities. The report addresses technological developments such as nanostructured carriers, AI-based diffusion modeling, and smart wearable patches, offering insights into operational efficiency, sustainability, and digital transformation trends. Emerging segments, including multi-layer sequential-release gels and biodegradable polymer formulations, are also examined.
Overall, the scope provides decision-makers with a clear understanding of market dynamics, competitive landscape, innovation trajectories, and strategic growth avenues across the global Prednisolone Transdermal Gel ecosystem.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2024) | USD 490 Million |
| Market Revenue (2032) | USD 675.8 Million |
| CAGR (2025–2032) | 4.1% |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User Insights
|
| Key Report Deliverables | Revenue Forecast, Growth Drivers & Restraints, Technology Insights, Market Dynamics, Segmentation Analysis, Regional Insights, Competitive Landscape, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Pfizer Inc., GlaxoSmithKline, Novartis AG, Sun Pharmaceutical Industries, Mylan N.V., Sanofi S.A., AstraZeneca PLC, Bayer AG |
| Customization & Pricing | Available on Request (10% Customization is Free) |
