Reports
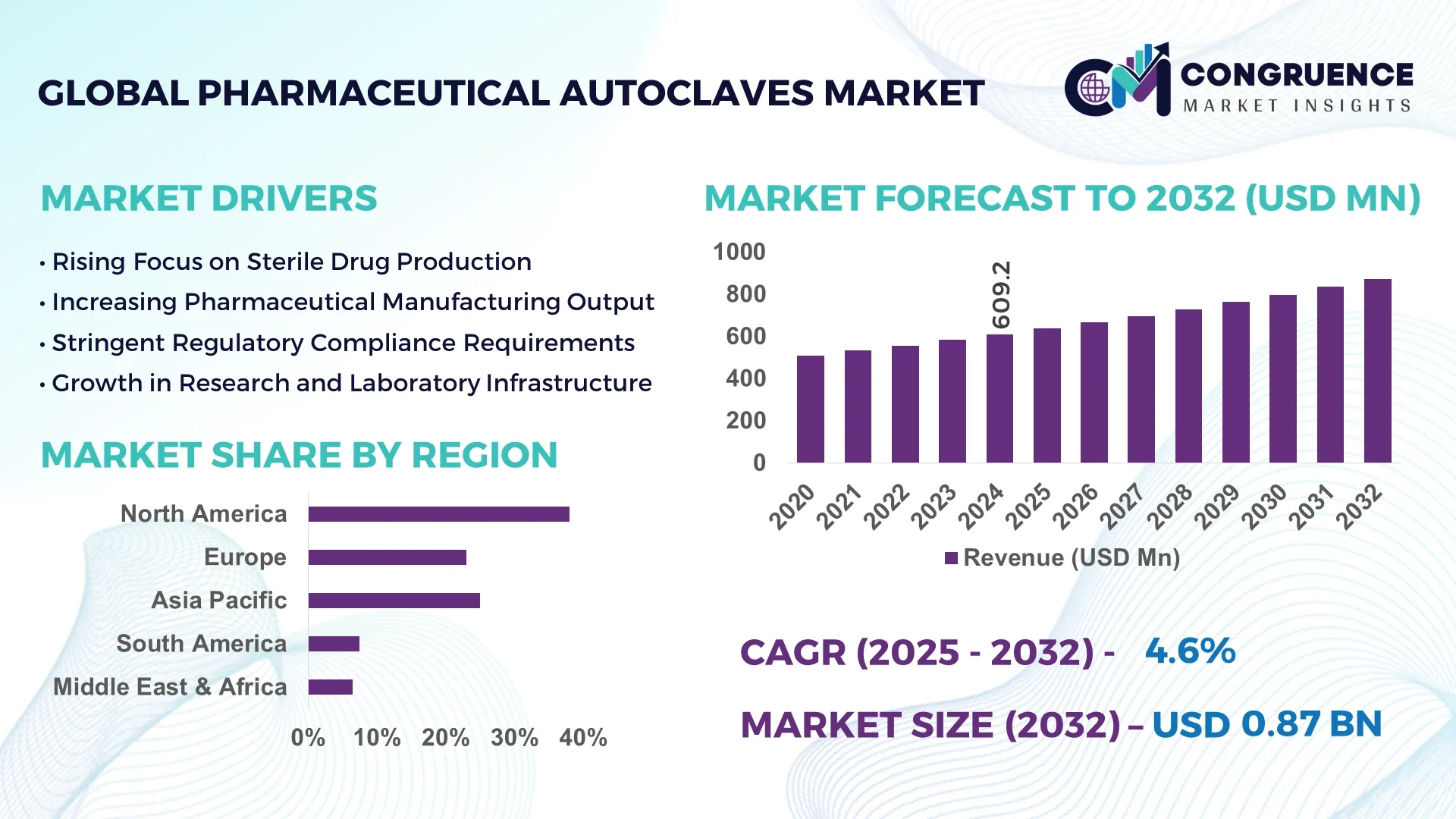
The Global Pharmaceutical Autoclaves Market was valued at USD 609.2 Million in 2024 and is anticipated to reach a value of USD 873.0 Million by 2032 expanding at a CAGR of 4.6% between 2025 and 2032.
To Learn More About This Report, Request A Free Sample Copy
Asia-Pacific dominates the pharmaceutical autoclaves market due to its rapidly growing pharmaceutical manufacturing sector, supported by increasing investments in sterile drug production and regulatory frameworks emphasizing sterilization standards. Countries like India and China have become hubs for pharmaceutical production, driving demand for advanced sterilization technologies such as autoclaves to ensure product safety and compliance.
The pharmaceutical autoclaves market is characterized by technological innovation, with companies focusing on developing automated, energy-efficient, and user-friendly sterilization equipment. Growing concerns over contamination and stringent quality control measures in drug manufacturing are boosting the demand for reliable sterilization solutions. Additionally, the expanding biologics and vaccine segments require advanced autoclave systems to handle complex sterilization needs, fueling market growth. Integration with digital monitoring and control systems is also enhancing operational efficiency and traceability in pharmaceutical sterilization processes globally.
Artificial intelligence (AI) is significantly transforming the pharmaceutical autoclaves market by enhancing process automation, improving sterilization precision, and enabling predictive maintenance. AI-powered systems can optimize cycle parameters in real-time, ensuring consistent sterilization outcomes while reducing energy consumption. Machine learning algorithms analyze vast amounts of operational data to detect anomalies and predict equipment failures before they occur, minimizing downtime and maintenance costs. Moreover, AI integration allows for enhanced traceability and compliance reporting, critical in pharmaceutical manufacturing where strict regulatory standards must be met.
The implementation of AI also supports remote monitoring capabilities, enabling operators to oversee autoclave performance from anywhere, facilitating quicker response times to process deviations. This technological advancement is pivotal in large-scale pharmaceutical plants where maintaining sterilization integrity directly impacts product safety and batch release times. AI-driven analytics contribute to continuous process improvement by identifying inefficiencies and suggesting optimal operational adjustments, thereby improving productivity.
Additionally, AI enables customization of sterilization cycles based on specific drug formulations or container types, reducing material stress and extending equipment lifespan. The ability to learn from historical data and adapt processes dynamically helps manufacturers meet evolving sterilization challenges posed by complex biopharmaceutical products. Collectively, these AI-driven innovations are fostering smarter, safer, and more efficient sterilization solutions in the pharmaceutical autoclaves market.
“In 2024, a breakthrough AI-based autoclave monitoring system was introduced that uses deep learning to detect microbial contamination risks during sterilization cycles, achieving detection accuracy above 95% and reducing validation time by 30%.”
The rising demand for sterile pharmaceutical products is a critical driver of the pharmaceutical autoclaves market. Increasing prevalence of chronic diseases and a growing global population are fueling pharmaceutical production volumes, thereby escalating the need for reliable sterilization methods. Sterilization is vital to ensuring the safety and efficacy of injectable drugs, vaccines, and biologics, which require contamination-free environments. Enhanced awareness regarding product safety among healthcare providers and patients is driving strict adherence to sterilization protocols, prompting pharmaceutical manufacturers to invest in advanced autoclave systems that guarantee high sterilization standards.
The pharmaceutical autoclaves market faces restraints due to the high initial costs associated with purchasing and installing advanced sterilization equipment. Additionally, ongoing maintenance expenses, including validation and calibration, can be substantial, particularly for smaller pharmaceutical companies with limited budgets. These financial barriers may delay or limit the adoption of cutting-edge autoclave technologies. Furthermore, the complexity of integrating new systems into existing manufacturing lines can pose operational challenges, deterring some companies from upgrading their sterilization infrastructure.
The rapid growth of the biopharmaceutical and vaccine sectors presents significant opportunities for the pharmaceutical autoclaves market. These segments demand highly specialized sterilization processes due to the sensitivity of biological products. Increasing government initiatives and funding to support vaccine manufacturing, especially in response to global health emergencies, are driving investments in sophisticated autoclave systems. Additionally, the rise of personalized medicine requires flexible and adaptable sterilization equipment capable of handling smaller batch sizes with high precision, opening avenues for market expansion.
Meeting the stringent regulatory compliance and validation requirements poses a major challenge in the pharmaceutical autoclaves market. Pharmaceutical manufacturers must adhere to rigorous sterilization protocols defined by global health authorities, which demand extensive documentation, frequent equipment validation, and process monitoring. These requirements can increase operational complexity and extend time-to-market for pharmaceutical products. Ensuring that autoclave systems consistently meet such standards requires continuous investment in staff training, equipment upgrades, and process optimization, which can strain resources.
Rise in Modular and Prefabricated Autoclave Designs: Modular and prefabricated autoclave systems are increasingly adopted for their ability to reduce installation time and costs. These designs allow for scalable, customizable sterilization solutions, accelerating deployment in pharmaceutical manufacturing facilities, especially in regions with expanding production capacities.
Integration of Digital Technologies: Real-time monitoring, remote diagnostics, and automated data logging features are becoming standard in autoclave systems. This digital transformation enhances process transparency, reduces human error, and ensures compliance with stringent pharmaceutical manufacturing regulations. IoT connectivity enables predictive maintenance and improved operational uptime.
Focus on Environmental Sustainability: Energy-efficient autoclaves that minimize water and electricity consumption without compromising sterilization effectiveness are gaining popularity. This trend aligns with the pharmaceutical industry's broader commitment to reducing environmental impact and promoting greener manufacturing practices.
Demand for Sterilization of Advanced Biopharmaceutical Formats: Autoclave systems capable of sterilizing complex biopharmaceutical products such as single-use systems and innovative drug delivery devices are in demand. These require specialized sterilization cycles that preserve product integrity while ensuring safety.
Growth of Compact and User-Friendly Systems for Small and Medium-Sized Facilities: There is increasing demand for compact, easy-to-operate autoclaves tailored for small to medium pharmaceutical labs and contract manufacturing organizations (CMOs). These systems emphasize quick cycle times, flexible loading, and user-friendly interfaces to meet diverse operational needs.
The pharmaceutical autoclaves market is segmented by type, application, and end-user to provide detailed insights into market dynamics. Types of autoclaves include gravity displacement, pre-vacuum, and steam pulsing, each serving distinct sterilization needs based on pressure and cycle requirements. Applications range from sterilizing surgical instruments, pharmaceutical manufacturing equipment, to laboratory tools and packaging materials. End-users comprise pharmaceutical companies, hospitals, research laboratories, and contract manufacturing organizations (CMOs). Understanding these segments helps identify which areas are driving market growth and how technological preferences vary across different sectors. This segmentation analysis is vital for manufacturers and stakeholders aiming to tailor their product offerings and strategic initiatives according to specific market demands.
The pharmaceutical autoclaves market is primarily divided into gravity displacement autoclaves, pre-vacuum autoclaves, and steam pulsing autoclaves. Among these, gravity displacement autoclaves lead the market due to their simplicity, cost-effectiveness, and widespread use in sterilizing heat-stable equipment and media. These autoclaves operate by displacing air through steam gravity, making them reliable for basic sterilization needs. However, the fastest-growing segment is the pre-vacuum autoclaves, which offer advanced sterilization by removing air prior to steam introduction, ensuring thorough penetration and sterility for complex instruments and pharmaceutical products. This segment's growth is propelled by increasing demand in biologics and vaccine production, where sterilization standards are more stringent. Steam pulsing autoclaves are gaining attention in specialized applications requiring controlled steam pulses for sensitive materials. Collectively, these types cater to varying pharmaceutical sterilization requirements, balancing cost and technological sophistication.
Pharmaceutical autoclaves find applications across sterilization of surgical instruments, pharmaceutical manufacturing equipment, laboratory tools, and packaging materials. The sterilization of pharmaceutical manufacturing equipment dominates the market due to the increasing production of sterile injectable drugs, vaccines, and biologics requiring reliable sterilization processes to maintain safety and efficacy. This application segment benefits from strict regulatory mandates necessitating validated sterilization cycles. The fastest-growing application segment is sterilization of laboratory tools and instruments, driven by the expansion of research and development activities in pharmaceuticals, biotechnology, and vaccine development. Laboratories require precise sterilization to prevent cross-contamination and ensure experimental accuracy. Sterilization of packaging materials is also witnessing growth, especially with the rise of aseptic packaging in sterile drug delivery systems, supporting longer shelf life and enhanced product safety.
End-users of pharmaceutical autoclaves include pharmaceutical manufacturing companies, hospitals, research laboratories, and contract manufacturing organizations (CMOs). Pharmaceutical manufacturing companies lead the market, accounting for the largest share due to the surge in sterile drug production and the necessity for high-throughput sterilization equipment. This segment is supported by ongoing pharmaceutical industry growth worldwide, particularly in emerging markets. The fastest-growing end-user segment is contract manufacturing organizations (CMOs), which are expanding rapidly as pharmaceutical companies outsource manufacturing to reduce costs and increase flexibility. CMOs require versatile, scalable autoclave systems to cater to multiple clients with varied sterilization needs. Hospitals and research laboratories represent steady demand segments, primarily driven by infection control protocols and research activity expansions, respectively. Each end-user category contributes uniquely to market development based on operational scale and sterilization complexity.
North America accounted for the largest market share at 38% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 5.2% between 2025 and 2032.
To Learn More About This Report, Request A Free Sample Copy
North America leads due to its advanced pharmaceutical manufacturing infrastructure, stringent regulatory standards, and high adoption of cutting-edge sterilization technologies. Meanwhile, Asia-Pacific’s growth is driven by rapid industrialization, increasing pharmaceutical production capacity, and supportive government initiatives in countries like China and India. Europe holds a significant share due to its established pharmaceutical industry and innovation in sterilization equipment, while regions such as South America and the Middle East & Africa are emerging markets with growing healthcare investments and expanding pharmaceutical sectors.
Innovation and Regulatory Push Drive Market Expansion
In North America, the pharmaceutical autoclaves market is driven by ongoing innovations focusing on automation and AI integration. The region's strong regulatory environment, including agencies demanding rigorous sterilization validation, fuels demand for sophisticated autoclave systems. The U.S. pharmaceutical industry’s high volume of sterile injectable drug production necessitates reliable sterilization equipment, contributing to the region's market dominance. Canada’s growing biotech sector also supports the need for advanced autoclaves. Additionally, increasing investments in upgrading manufacturing facilities with digital monitoring capabilities are key trends observed across the region.
Sustainability and Technological Sophistication Shape Market
Europe’s pharmaceutical autoclaves market emphasizes sustainability and energy-efficient technologies. Countries such as Germany, France, and the UK focus on developing autoclaves that reduce water and energy consumption to meet strict environmental standards. The region’s mature pharmaceutical industry is adopting modular autoclave systems to streamline production and enhance flexibility. Increasing biopharmaceutical manufacturing, especially in the Nordic countries, is driving demand for specialized sterilization solutions. Additionally, Europe sees rising interest in autoclaves integrated with IoT for predictive maintenance, ensuring consistent sterilization quality and regulatory compliance.
Rapid Industrial Growth and Investment Surge
Asia-Pacific is experiencing a surge in pharmaceutical manufacturing, with countries like China, India, and Japan leading the expansion. The market growth is fueled by rising healthcare demands, increasing production of vaccines and biologics, and government support for pharmaceutical infrastructure development. There is growing adoption of automated autoclave systems capable of handling large batch sizes to meet escalating production needs. The region also benefits from increasing foreign direct investments and the presence of numerous contract manufacturing organizations requiring versatile sterilization equipment. Efforts to align with global quality standards are further boosting the market.
Emerging Market Expansion and Infrastructure Development
South America’s pharmaceutical autoclaves market is developing steadily, with Brazil and Argentina as key contributors. Growing investments in healthcare infrastructure and increasing pharmaceutical manufacturing activities are driving demand for reliable sterilization equipment. The expansion of generic drug production is a notable trend, requiring efficient autoclave systems to maintain product safety. Additionally, efforts to modernize existing manufacturing facilities with energy-efficient and digitally connected autoclaves are gaining traction. Regional regulatory improvements are encouraging adoption of advanced sterilization technologies across the continent.
Healthcare Modernization and Rising Pharmaceutical Activities
The Middle East & Africa region is witnessing growing pharmaceutical manufacturing activities, particularly in the UAE and South Africa, driving the need for advanced autoclaves. Government initiatives aimed at healthcare modernization and pharmaceutical sector development are key market drivers. Increasing adoption of modular autoclaves enables quick deployment in emerging markets with evolving infrastructure. The region’s focus on reducing import dependence by boosting local pharmaceutical production is expanding the demand for sterilization equipment. Additionally, rising awareness of contamination control and compliance is promoting market growth.
United States: USD 230 Million – Due to advanced pharmaceutical manufacturing infrastructure and strong regulatory environment.
China: USD 150 Million – Driven by rapid pharmaceutical industrial growth and government incentives supporting sterile drug production.
The pharmaceutical autoclaves market is highly competitive, with several established players focusing on technological innovation, product quality, and expanding their geographical footprint. Key companies are investing heavily in R&D to introduce advanced autoclave systems equipped with automation, real-time monitoring, and energy-saving features. Market leaders hold significant shares by leveraging strong distribution networks and comprehensive after-sales services. New entrants are focusing on niche applications and customizable solutions to capture specific market segments. Collaborations, partnerships, and strategic acquisitions are common strategies used to enhance product portfolios and regional presence. The competition also extends to price differentiation, as manufacturers aim to offer cost-effective sterilization solutions without compromising performance. Product differentiation through digital integration and eco-friendly designs is becoming a critical factor in gaining competitive advantage.
Getinge AB
Steris Plc
Tuttnauer Europe B.V.
Belimed AG
Fedegari Group
MMM Group
Andersen Products, Inc.
Astell Scientific Ltd
STERIS AST
Priorclave Ltd
Technological advancements in pharmaceutical autoclaves are shaping the market by improving sterilization efficacy and operational efficiency. Modern autoclaves incorporate advanced sensors and microprocessor controls to monitor temperature, pressure, and sterilization cycles with high precision. Innovations include pre-vacuum technology that ensures enhanced steam penetration for complex instruments and porous loads. The integration of IoT enables remote monitoring and predictive maintenance, reducing downtime and operational risks. Additionally, development of eco-friendly autoclaves focusing on energy conservation and water recycling is gaining momentum to align with sustainability goals. Steam pulsing technology offers sterilization for heat-sensitive materials by alternating steam and vacuum cycles. Moreover, user interfaces are becoming more intuitive, allowing operators to customize and validate sterilization processes easily. These technological improvements ensure compliance with stringent regulatory standards while optimizing throughput and safety.
In September 2024, Fedegari launched its new generation of steam sterilizers featuring AI-powered process optimization, enhancing sterilization cycle efficiency and reducing energy consumption by 18%.
In November 2023, Steris Plc unveiled a next-gen autoclave system with integrated IoT capabilities for remote diagnostics and automated maintenance alerts, improving operational uptime in pharmaceutical manufacturing plants.
In April 2024, Getinge AB expanded its pharmaceutical sterilization equipment portfolio by introducing modular autoclaves that allow quick on-site installation and scalability tailored to varying production demands.
In August 2023, Tuttnauer Europe B.V. announced the release of a compact autoclave designed for small to medium pharmaceutical laboratories, featuring rapid cycle times and digital data logging for enhanced traceability.
The pharmaceutical autoclaves market report comprehensively covers product types, applications, end-user industries, and geographical regions, providing detailed insights into current market trends and future opportunities. It addresses the demand for sterilization equipment in pharmaceutical manufacturing, hospital sterilization departments, research laboratories, and contract manufacturing organizations. The report examines the impact of regulatory frameworks on sterilizer adoption and highlights technological innovations such as automation, digital monitoring, and eco-friendly systems. It also includes competitive analysis, profiling key market players and their strategic initiatives. With detailed segmentation by type (gravity displacement, pre-vacuum, steam pulsing), application (instrument sterilization, packaging, laboratory equipment), and end-users, the report aids stakeholders in understanding market dynamics. Additionally, regional analysis focuses on growth drivers and challenges in North America, Europe, Asia-Pacific, South America, and the Middle East & Africa, facilitating informed decision-making for manufacturers, investors, and policymakers.
| Report Attribute / Metric | Report Details |
|---|---|
| Market Name | Global Pharmaceutical Autoclaves Market |
| Market Revenue (2024) | USD 609.2 Million |
| Market Revenue (2032) | USD 873.0 Million |
| CAGR (2025–2032) | 4.6% |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User
|
| Key Report Deliverables | Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape, Technological Insights, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Getinge AB, Steris Plc, Tuttnauer Europe B.V., Belimed AG, Fedegari Group, MMM Group, Andersen Products, Inc., Astell Scientific Ltd, STERIS AST, Priorclave Ltd |
| Customization & Pricing | Available on Request (10% Customization is Free) |
