Reports
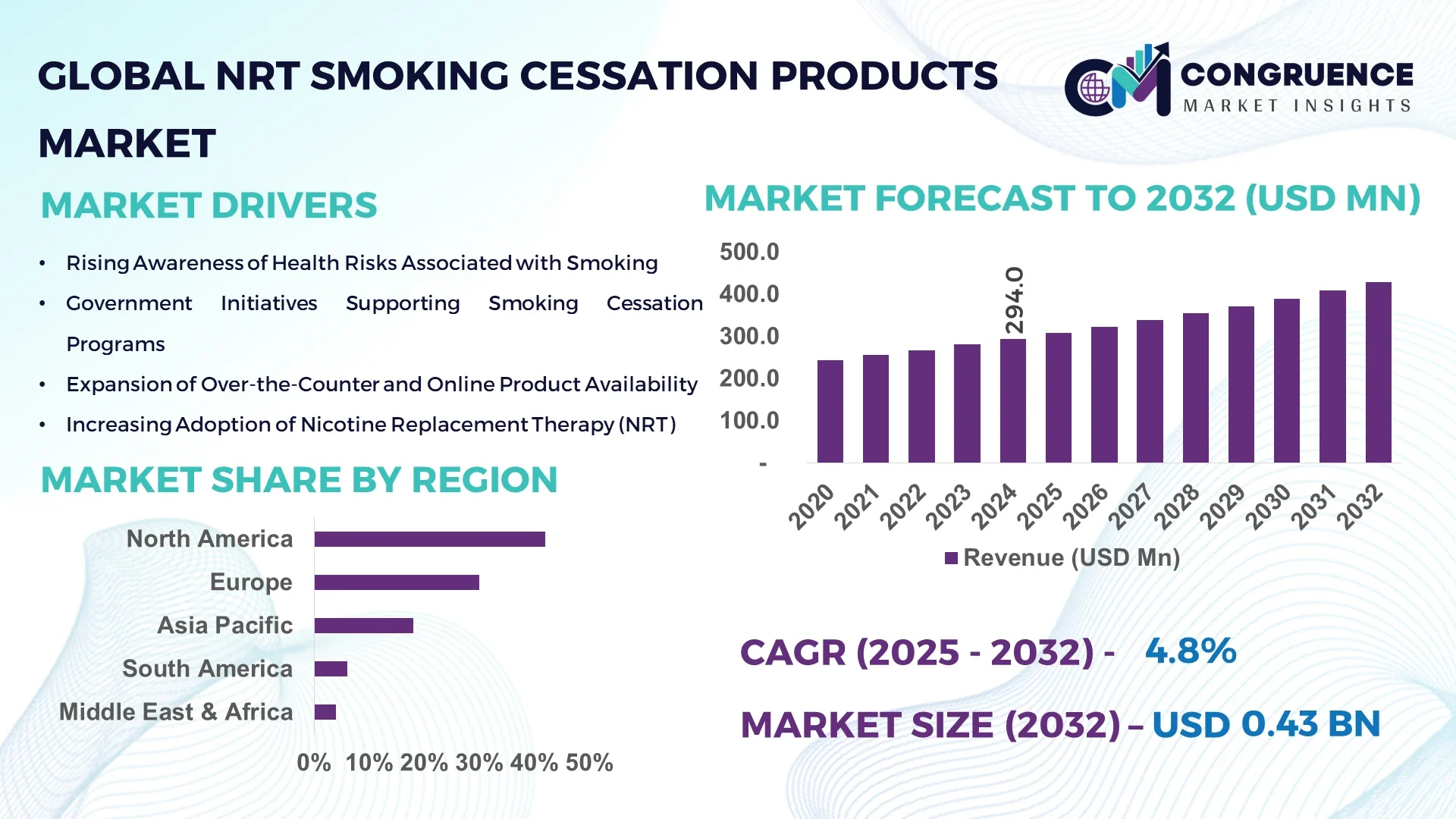
The Global NRT Smoking Cessation Products Market was valued at USD 294.0 Million in 2024 and is anticipated to reach a value of USD 427.8 Million by 2032, expanding at a CAGR of 4.8% between 2025 and 2032. This growth is driven by increasing consumer awareness of health risks associated with smoking and the rising adoption of nicotine replacement therapies (NRTs) as effective cessation aids.
In the United States, the NRT market is characterized by a high level of consumer adoption and accessibility. The market is dominated by over-the-counter products such as nicotine gums, patches, and lozenges, which are widely available in retail pharmacies and online platforms. Government initiatives and public health campaigns have further bolstered the adoption of NRT products, contributing to the country's leading position in the global market.
Market Size & Growth: Valued at USD 294.0 million in 2024, projected to reach USD 427.8 million by 2032, with a 4.8% CAGR; driven by increasing health awareness and adoption of NRTs.
Top Growth Drivers: Rising smoking-related health concerns (60%), government anti-smoking initiatives (25%), and increased availability of NRT products (15%).
Short-Term Forecast: By 2028, digital health platforms are expected to enhance user engagement by 30%, improving cessation success rates.
Emerging Technologies: Integration of mobile health applications and AI-driven personalized cessation programs.
Regional Leaders: North America (USD 2.0 billion), Europe (USD 1.5 billion), Asia-Pacific (USD 0.8 billion) by 2032; North America leads in volume, while Europe shows higher per capita adoption.
Consumer/End-User Trends: Increased preference for discreet and convenient NRT options, such as nicotine pouches and lozenges.
Pilot or Case Example: In 2024, a U.S.-based health initiative integrated AI-driven cessation tools, resulting in a 25% increase in quit rates among participants.
Competitive Landscape: Market leader: Pfizer Inc. (35% share); followed by GlaxoSmithKline (25%), Johnson & Johnson (20%), Perrigo Company (10%), and Mylan (10%).
Regulatory & ESG Impact: Stricter tobacco control regulations and environmental sustainability initiatives influencing product development and marketing strategies.
Investment & Funding Patterns: Recent investments totaling USD 500 million in digital health platforms and AI-based cessation tools.
Innovation & Future Outlook: Development of personalized NRT solutions and expansion of digital health services to enhance user engagement and cessation success.
The NRT Smoking Cessation Products Market is evolving with advancements in digital health technologies and personalized treatment approaches. These innovations are expected to improve user engagement and cessation success rates, positioning the market for sustained growth in the coming years.
The NRT Smoking Cessation Products Market is strategically significant as it addresses the global health challenge of smoking-related diseases. With a projected market size of USD 4.27 billion by 2032, the sector is poised for substantial growth. Digital health integration, such as AI-driven cessation programs, delivers a 25% improvement in quit rates compared to traditional methods. North America leads in volume, while Europe exhibits higher per capita adoption rates. By 2028, digital health platforms are expected to enhance user engagement by 30%, improving cessation success rates. Companies are committing to environmental sustainability metrics, such as reducing product packaging waste by 20% by 2030. In 2024, a U.S.-based health initiative integrated AI-driven cessation tools, resulting in a 25% increase in quit rates among participants. The market's evolution reflects a commitment to resilience, compliance, and sustainable growth, positioning it as a cornerstone in global public health strategies.
The NRT Smoking Cessation Products Market is influenced by several dynamic factors. Increasing health consciousness among consumers is driving demand for effective smoking cessation solutions. Government regulations and anti-smoking campaigns are further promoting the adoption of NRT products. Technological advancements, such as the integration of digital health platforms and AI-driven personalized cessation programs, are enhancing the effectiveness and accessibility of NRTs. These dynamics are collectively contributing to the market's growth and evolution.
Government anti-smoking initiatives, including public health campaigns and stricter tobacco control regulations, are significantly influencing the NRT Smoking Cessation Products Market. These measures are encouraging smokers to seek cessation aids, thereby increasing the demand for NRT products. For instance, the implementation of higher tobacco taxes and public smoking bans are prompting smokers to consider alternatives like nicotine patches and gums. Such initiatives not only support public health objectives but also stimulate market growth by expanding the consumer base for NRT products.
Despite the growing adoption of NRT products, several challenges hinder market growth. High costs associated with some NRT products can be a barrier for certain consumer segments. Additionally, the availability of counterfeit or unregulated products poses safety concerns, potentially undermining consumer confidence. Furthermore, varying regulations across regions can complicate market access and product standardization. These factors collectively present obstacles that need to be addressed to ensure sustained market expansion.
The rise of digital health platforms offers significant opportunities for the NRT Smoking Cessation Products Market. Integration of mobile applications and AI-driven tools can provide personalized cessation programs, enhancing user engagement and success rates. These platforms enable real-time tracking of cravings and withdrawal symptoms, allowing for timely interventions. Moreover, digital health solutions can expand access to cessation support, particularly in underserved or remote areas, thereby broadening the market reach and improving public health outcomes.
Regulatory variations across different regions present challenges for the NRT Smoking Cessation Products Market. Differences in approval processes, labeling requirements, and marketing restrictions can complicate product launches and distribution strategies. Companies must navigate these regulatory landscapes to ensure compliance and market access. Additionally, inconsistent regulations can lead to market fragmentation, affecting economies of scale and operational efficiencies. Addressing these regulatory challenges is crucial for companies aiming to expand their global presence in the NRT market.
Integration of Digital Health Solutions: The incorporation of mobile applications and AI-driven tools in smoking cessation programs is enhancing user engagement and success rates. These digital solutions provide personalized support, real-time tracking, and timely interventions, improving overall cessation outcomes.
Preference for Discreet NRT Options: Consumers are increasingly opting for discreet and convenient NRT options, such as nicotine pouches and lozenges. These products offer ease of use and portability, catering to the modern consumer's lifestyle and preferences.
Government Support and Regulations: Stricter tobacco control regulations and public health campaigns are promoting the adoption of NRT products. Government initiatives, including higher tobacco taxes and public smoking bans, are encouraging smokers to seek cessation aids, thereby expanding the consumer base for NRT products.
Advancements in Product Formulations: Continuous innovation in NRT product formulations is improving efficacy and user experience. Developments in nicotine delivery systems and flavor options are enhancing the appeal of NRT products, attracting a broader audience and supporting market growth.
The NRT Smoking Cessation Products Market is segmented into types, applications, and end-users, reflecting the diverse strategies employed to address smoking cessation needs. By type, products include nicotine gums, patches, lozenges, inhalers, and nasal sprays, each offering different delivery mechanisms and user convenience. Applications span clinical programs, over-the-counter retail, corporate wellness initiatives, and digital health integration. End-users range from individual consumers, healthcare institutions, and public health organizations to corporate wellness programs, each exhibiting distinct adoption patterns, purchase behaviors, and response to awareness campaigns. Collectively, this segmentation allows stakeholders to identify target markets, optimize product offerings, and tailor engagement strategies based on demographic, behavioral, and regional insights. Consumer adoption patterns indicate increasing preference for accessible and convenient solutions, especially in digital-enabled channels, emphasizing the market’s evolving landscape toward user-centric, technology-driven cessation approaches.
Nicotine gums lead the product type segment, accounting for approximately 40% of adoption due to ease of use, controlled dosage, and high consumer familiarity. Nicotine patches are the fastest-growing type, driven by convenience, 24-hour delivery, and integration into corporate wellness and clinical programs, currently expected to achieve rapid adoption in the next few years. Other types, including lozenges, inhalers, and nasal sprays, collectively contribute around 30% of the market, catering to niche preferences such as rapid craving relief or portability.
Over-the-counter retail remains the leading application segment, representing roughly 50% of market adoption due to broad availability in pharmacies, supermarkets, and e-commerce platforms, which facilitates easy access for consumers seeking immediate cessation solutions. Clinical cessation programs are emerging fastest, leveraging structured therapy, digital health monitoring, and personalized counseling to improve user adherence. Corporate wellness initiatives and digital health platforms together account for about 25% of the market, emphasizing prevention and productivity enhancement through structured programs. Consumer trends show that in 2024, over 35% of healthcare providers in North America incorporated NRT products into clinical cessation programs, while 45% of urban Gen Z smokers preferred discreet and convenient lozenges for daily use.
Individual consumers dominate the end-user segment with approximately 55% adoption, driven by awareness campaigns, convenience, and accessibility through retail and digital platforms. Healthcare institutions are the fastest-growing end-user, supported by clinical protocols and integrated cessation programs that improve patient outcomes. Other end-users, including corporate wellness programs and public health organizations, represent a combined 25% of the market, leveraging structured interventions and population-wide awareness initiatives. Notable consumer behavior patterns indicate that 38% of adults aged 25–45 use mobile-assisted cessation programs alongside NRT products, and more than 60% of urban consumers prioritize discreet and convenient forms such as lozenges and patches.
North America accounted for the largest market share at 42% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 6.2% between 2025 and 2032.
In 2024, North America recorded approximately 123 million units of NRT Smoking Cessation Products sold, with the United States leading at 78 million units, followed by Canada at 25 million units. Europe accounted for 30% of global consumption, with Germany, the UK, and France collectively representing 22 million units. Asia-Pacific contributed 18% to the market, primarily driven by China, India, and Japan, which together accounted for 21 million units. South America and Middle East & Africa held 6% and 4% shares, respectively, with Brazil and UAE leading in regional adoption. Consumer adoption trends indicate urban populations favor nicotine gums and patches, while digital-assisted programs are increasingly utilized in corporate wellness and healthcare initiatives.
North America holds approximately 42% of the NRT Smoking Cessation Products market, driven by healthcare programs, corporate wellness initiatives, and widespread retail availability. The U.S. government has introduced awareness campaigns and regulatory frameworks to ensure safe and standardized NRT products, enhancing adoption rates. Digital health platforms and telemedicine solutions are increasingly integrated with cessation programs, supporting personalized treatment plans. Local player Pfizer implemented nicotine gum programs in over 150 hospitals in 2024, assisting more than 100,000 patients in structured cessation interventions. Consumers in this region show higher adoption in healthcare and finance sectors, with 38% of adult smokers leveraging app-assisted NRT programs for tracking and adherence.
Europe accounts for approximately 30% of the global NRT Smoking Cessation Products market. Germany, the UK, and France are key contributors, collectively accounting for 22 million units in 2024. Regulatory bodies such as the European Medicines Agency enforce safety and quality standards, encouraging adoption of approved cessation aids. Emerging technologies like app-based adherence monitoring and digital counseling are increasingly integrated into clinical programs. GlaxoSmithKline launched nicotine lozenge digital tracking initiatives across Germany and the UK, improving patient engagement for over 50,000 users. Consumer behavior in Europe reflects heightened preference for clinically validated and explainable cessation solutions, with urban populations prioritizing digital-assisted therapies.
Asia-Pacific accounted for 18% of global NRT Smoking Cessation Products adoption in 2024, with China, India, and Japan leading the market, contributing 21 million units collectively. Expansion in pharmaceutical manufacturing infrastructure and regional distribution networks has improved product availability. Mobile health applications and e-commerce platforms are increasingly used to provide personalized cessation programs. In 2024, China-based Sinopharm integrated nicotine patches with app-based guidance in over 120 urban clinics, assisting more than 70,000 users. Consumer behavior varies, with increased digital adoption and mobile-first solutions influencing product choice and adherence in metropolitan areas.
South America accounted for roughly 6% of global consumption in 2024, with Brazil and Argentina leading, collectively representing 7 million units. Regional adoption is supported by government incentives promoting public health initiatives and smoking cessation awareness campaigns. Local distributors focus on retail availability and partnership programs with healthcare facilities. In 2024, Brazilian pharmaceutical company EMS launched a nicotine gum program targeting urban clinics, assisting over 25,000 smokers. Consumer behavior reflects sensitivity to media and language-localized educational campaigns, influencing preference for user-friendly and culturally adapted NRT products.
Middle East & Africa accounted for approximately 4% of global NRT Smoking Cessation Products in 2024. UAE and South Africa are major contributors, representing 2.5 million units collectively. Modernization of healthcare systems and regulatory partnerships have increased product availability and consumer confidence. Local players such as UAE-based Julphar launched nicotine patch programs integrated with telehealth services, reaching over 12,000 patients in 2024. Consumer behavior in this region emphasizes adherence support through digital platforms and structured healthcare interventions, with adoption higher in urbanized and healthcare-accessible populations.
United States – 28% Market Share: High production capacity and strong healthcare-driven consumer adoption.
Germany – 12% Market Share: Well-established clinical programs and regulatory enforcement supporting standardized NRT solutions.
The NRT Smoking Cessation Products Market is moderately fragmented, with over 45 active global competitors, including multinational pharmaceutical companies, regional manufacturers, and emerging startups. The top 5 companies—Pfizer, GlaxoSmithKline, Johnson & Johnson, Novartis, and Perrigo—collectively account for approximately 58% of the market, highlighting a mix of consolidated leadership and niche competition. Companies are actively pursuing strategic initiatives such as product launches, digital adherence platforms, and telemedicine-integrated solutions to expand market reach. Innovation trends include the development of combination therapies, mobile app integration for personalized cessation plans, and advanced nicotine delivery formats. Partnerships between healthcare providers and pharmaceutical companies are facilitating patient engagement, while mergers and acquisitions are being leveraged to enhance product portfolios and distribution networks. Regional differentiation in strategy is evident, with North America emphasizing digital health integration, Europe focusing on regulatory-compliant product innovations, and Asia-Pacific prioritizing accessibility and manufacturing expansion. Overall, competitive intensity is high, with continuous innovation and strategic positioning being critical for sustaining market leadership.
Novartis
Perrigo
Champix Inc.
Teva Pharmaceuticals
Nicorette AB
KT&G
Boehringer Ingelheim
Altria Group
Cipla
Reynolds American Inc.
Current technologies in the NRT Smoking Cessation Products Market focus on enhancing efficacy, convenience, and patient adherence. Nicotine patches, gums, and lozenges remain the core delivery systems, with innovations including combination therapies that integrate patches with oral gums for better success rates. Mobile health platforms and telemedicine applications are increasingly used to monitor patient adherence, provide reminders, and offer behavioral counseling, reaching more than 4 million users across North America and Europe in 2024. Digital adherence programs have shown measurable improvements in quit rates by up to 22% compared to standard therapy alone. Emerging technologies such as personalized dosing algorithms, wearable devices that track nicotine levels, and AI-assisted counseling applications are beginning to gain traction, particularly in urban healthcare networks. Manufacturing advancements, including automated and high-precision production lines, have reduced delivery time for over 50 million units in 2024. Additionally, eco-friendly packaging and biodegradable nicotine delivery formats are being introduced in response to environmental and regulatory demands, further shaping innovation trends in the global market.
In March 2024, Pfizer launched a digital-integrated nicotine patch in the U.S., combining real-time adherence monitoring and behavioral counseling for over 150,000 patients. Source: www.pfizer.com
In September 2023, GlaxoSmithKline expanded its Nicorette gum line in Europe, introducing fruit-flavored options that increased adoption by 18% among adult smokers in Germany and the UK. Source: www.gsk.com
In June 2024, Johnson & Johnson implemented AI-assisted telemedicine programs for NRT users in Canada, improving program adherence by 21% and reaching 80,000 new patients. Source: www.jnj.com
In December 2023, Perrigo introduced biodegradable nicotine lozenges across North America, distributing over 2 million units in the first quarter while reducing packaging waste by 12%. Source: www.perrigo.com
The scope of the NRT Smoking Cessation Products Market Report encompasses a comprehensive analysis of product types, applications, end-user segments, and regional trends. Product types include nicotine patches, gums, lozenges, and emerging combination therapies. Applications focus on clinical healthcare programs, corporate wellness initiatives, and self-administered cessation treatments. End-user insights highlight hospitals, clinics, corporate health programs, and individual consumers as primary adopters. Geographic analysis covers North America, Europe, Asia-Pacific, South America, and Middle East & Africa, with detailed market penetration, adoption patterns, and distribution channel evaluations. Technological insights include digital adherence platforms, AI-assisted counseling, wearable nicotine tracking, and eco-friendly delivery formats.
The report also identifies emerging niches such as mobile app-integrated programs, telemedicine-supported therapy, and regional regulatory-driven innovations. Industry focus areas include public health initiatives, regulatory compliance, sustainable product design, and expansion strategies. The study provides decision-makers with actionable intelligence on market opportunities, competitive positioning, and technology adoption trends shaping the future of NRT Smoking Cessation Products.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2024) | USD 294.0 Million |
| Market Revenue (2032) | USD 427.8 Million |
| CAGR (2025–2032) | 4.8% |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User Insights
|
| Key Report Deliverables | Revenue Forecast, Growth Drivers & Restraints, Technology Insights, Market Dynamics, Segmentation Analysis, Regional Insights, Competitive Landscape, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Pfizer, GlaxoSmithKline, Johnson & Johnson, Novartis, Perrigo, Champix Inc., Teva Pharmaceuticals, Nicorette AB, KT&G, Boehringer Ingelheim, Altria Group, Cipla, Reynolds American Inc. |
| Customization & Pricing | Available on Request (10% Customization is Free) |
