Reports
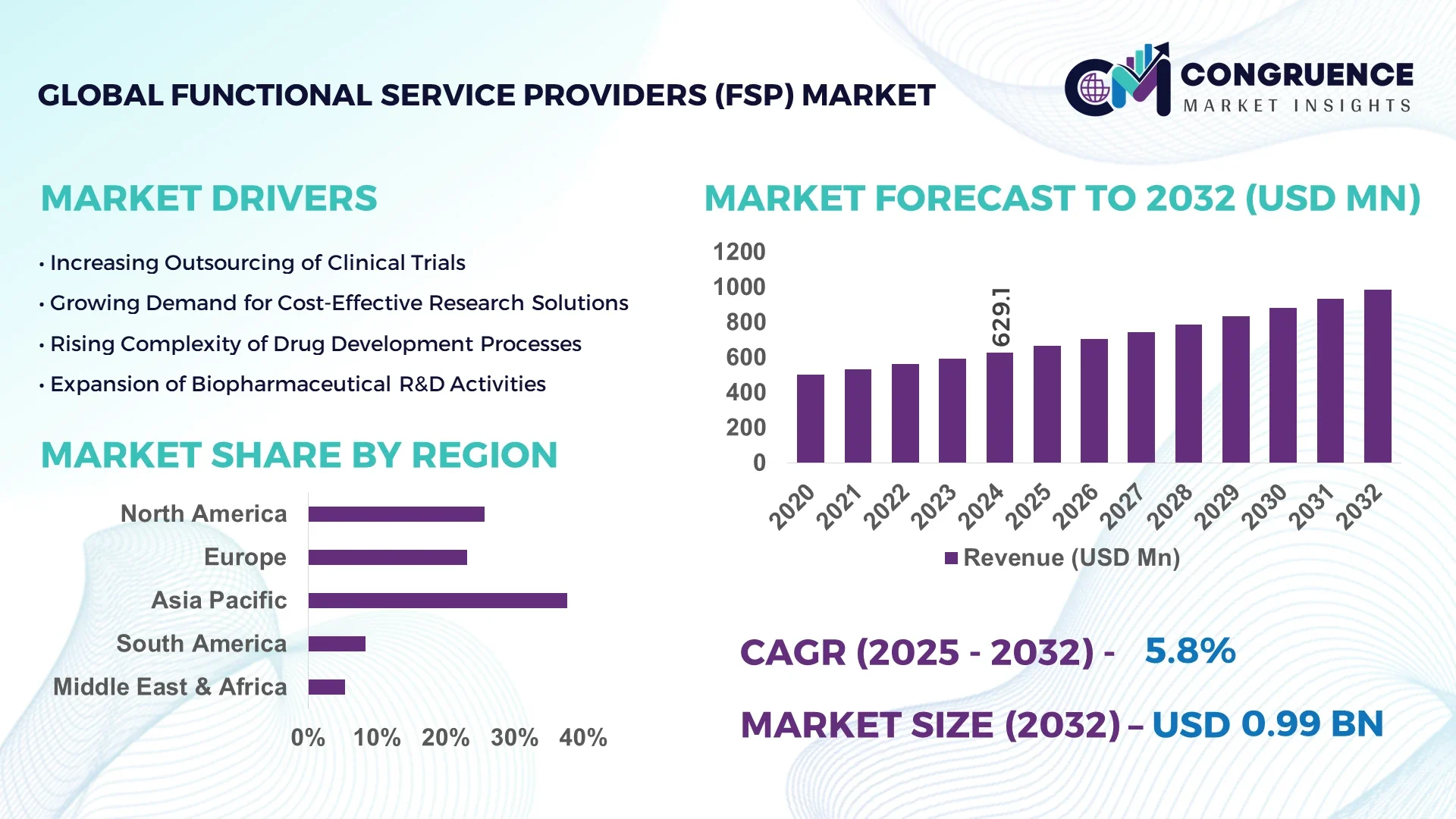
The Global Functional Service Providers (FSP) Market was valued at USD 629.08 Million in 2024 and is anticipated to reach a value of USD 987.62 Million by 2032, expanding at a CAGR of 5.8% between 2025 and 2032. This growth is primarily driven by the increasing demand for specialized services in clinical trials and the growing trend of outsourcing non-core functions to enhance operational efficiency.
The United States stands as a pivotal player in the global FSP market, underpinned by its substantial investment in research and development, particularly within the biopharmaceutical sector. In 2025, U.S. biopharmaceutical companies are projected to invest over USD 160 billion in R&D activities, reflecting a robust commitment to innovation and advancement. This investment fosters the development and adoption of cutting-edge technologies, such as AI-driven analytics and advanced data management systems, which are integral to the FSP model. Additionally, the U.S. regulatory environment supports the growth of FSPs, providing a conducive framework for clinical trials and related services. The nation's extensive infrastructure and expertise further solidify its leadership in the FSP market.
Market Size & Growth: Valued at USD 629.08 Million in 2024, projected to reach USD 987.62 Million by 2032, with a CAGR of 5.8% due to increased outsourcing in clinical trials.
Top Growth Drivers: Enhanced operational efficiency (45%), cost reduction in clinical trials (35%), and access to specialized expertise (20%).
Short-Term Forecast: By 2028, a 15% improvement in cost efficiency and a 20% reduction in trial timelines are anticipated.
Emerging Technologies: Integration of AI and machine learning for data analysis, adoption of blockchain for data security, and utilization of wearable devices for real-time patient monitoring.
Regional Leaders: North America: USD 450 Million by 2032; Europe: USD 300 Million by 2032; Asia-Pacific: USD 150 Million by 2032. North America leads in technological adoption, Europe focuses on regulatory compliance, and Asia-Pacific shows rapid growth in clinical trial outsourcing.
Consumer/End-User Trends: Biopharmaceutical companies increasingly favor FSP models for flexibility, while biotech firms seek specialized services to accelerate drug development.
Pilot or Case Example: In 2023, a U.S.-based FSP partnership led to a 25% reduction in clinical trial duration and a 30% decrease in operational costs.
Competitive Landscape: Leading provider: 25% market share; followed by three major competitors with 15%, 12%, and 10% shares, respectively.
Regulatory & ESG Impact: Stringent data protection regulations and environmental sustainability initiatives are shaping FSP operations, emphasizing compliance and ethical practices.
Investment & Funding Patterns: Recent investments totaling USD 500 million focus on technological advancements and infrastructure development in the FSP sector.
Innovation & Future Outlook: The future of FSPs lies in the integration of advanced technologies, expansion into emerging markets, and the development of tailored solutions to meet specific client needs.
The FSP market is experiencing significant growth, driven by the increasing complexity of clinical trials and the need for specialized services. Key industry sectors such as clinical trial management, data analytics, and regulatory affairs are witnessing enhanced service offerings through technological innovations and strategic partnerships. The adoption of AI and machine learning is revolutionizing data analysis, enabling more accurate and timely decision-making. Regulatory frameworks are evolving to accommodate the dynamic nature of clinical trials, ensuring compliance and patient safety. Regionally, North America continues to lead in technological advancements and infrastructure, while Asia-Pacific is emerging as a hub for clinical trial outsourcing due to cost advantages and a growing patient pool. Looking ahead, the FSP market is poised for continued expansion, with a focus on innovation, efficiency, and global collaboration.
The Functional Service Providers (FSP) market plays a pivotal role in the global healthcare and life sciences sectors by offering specialized outsourcing solutions that enhance operational efficiency and innovation. As organizations increasingly focus on core competencies, the demand for FSPs has surged, driven by the need for expertise in areas such as clinical trials, data management, and regulatory affairs. For instance, the integration of artificial intelligence (AI) in data analytics has demonstrated a 30% improvement in data processing speeds compared to traditional methods. Regionally, North America dominates in volume, while Asia-Pacific leads in adoption, with over 60% of enterprises in countries like India and China utilizing FSP models for clinical research and data management. In the short term, by 2027, the implementation of AI-driven analytics is expected to reduce clinical trial timelines by 20%, enhancing overall efficiency. From a compliance perspective, firms are committing to sustainability metrics such as a 25% reduction in carbon emissions by 2030, aligning with global environmental standards. A micro-scenario illustrating this trend is the collaboration between a leading U.S.-based FSP and a European pharmaceutical company in 2024, which resulted in a 15% reduction in trial costs through the adoption of cloud-based data management systems. Looking forward, the FSP market is poised to be a cornerstone of resilience, compliance, and sustainable growth, driven by technological advancements and a commitment to operational excellence.
The Functional Service Providers (FSP) market is undergoing significant transformation, influenced by technological advancements, regulatory changes, and shifting industry needs. Key dynamics include the increasing complexity of clinical trials, the need for specialized expertise, and the growing emphasis on cost-effective outsourcing solutions. These factors are reshaping the landscape, prompting organizations to seek flexible and scalable service models that can adapt to evolving demands. As a result, FSPs are expanding their service offerings, integrating advanced technologies, and focusing on compliance to meet the diverse needs of the healthcare and life sciences sectors.
The escalating complexity of clinical trials, characterized by multi-center studies, adaptive designs, and the inclusion of diverse patient populations, is significantly driving the demand for specialized services. FSPs offer tailored solutions in areas such as clinical monitoring, data management, and regulatory affairs, enabling sponsors to navigate these complexities efficiently. For example, the adoption of decentralized clinical trials has streamlined patient recruitment and data collection processes, enhancing trial efficiency and reducing operational costs.
Data security and privacy concerns remain significant challenges for the FSP market, particularly in handling sensitive patient information. Compliance with stringent regulations like the General Data Protection Regulation (GDPR) and the Health Insurance Portability and Accountability Act (HIPAA) necessitates robust data protection measures. Instances of data breaches or non-compliance can lead to legal repercussions and damage to reputation, thereby hindering the adoption of FSP models.
The rise of personalized medicine offers significant opportunities for FSPs to provide specialized services tailored to individual patient profiles. This includes the development of companion diagnostics, biomarker analysis, and customized treatment regimens. FSPs with expertise in genomics and molecular biology are well-positioned to support the growing demand for personalized therapeutic solutions, thereby expanding their service portfolios and market reach.
Regulatory complexities pose substantial challenges for FSPs, especially when operating across multiple jurisdictions with varying compliance requirements. Navigating these complex regulatory landscapes demands significant resources and expertise, potentially leading to delays in project timelines and increased operational costs. For instance, discrepancies in regulatory standards between regions can complicate the approval processes for clinical trials, affecting the overall efficiency of FSP operations.
Modular and Prefabricated Construction Adoption: The adoption of modular construction is reshaping demand dynamics in the Functional Service Providers (FSP) market. Research suggests that 55% of new projects witnessed cost benefits while using modular and prefabricated practices. Pre-bent and cut elements are prefabricated off-site using automated machines, reducing labor needs and speeding project timelines. Demand for high-precision machines is rising, especially in Europe and North America, where construction efficiency is critical.
Integration of Artificial Intelligence in Data Management: Artificial intelligence (AI) is increasingly integrated into data management processes within the FSP market. AI-driven analytics enhance data accuracy and processing speed, leading to more informed decision-making. This integration supports the growing need for real-time data analysis and predictive modeling in clinical trials and research.
Shift Towards Hybrid Outsourcing Models: There is a notable shift towards hybrid outsourcing models combining Functional Service Provider (FSP) and Full-Service Outsourcing (FSO) approaches. This hybrid model offers flexibility and scalability, allowing organizations to tailor services to specific project needs. It enables better resource allocation and risk management, enhancing overall project efficiency.
Emphasis on Regulatory Compliance and Sustainability: Regulatory compliance and sustainability have become central to the FSP market. Companies are adopting green building standards and sustainable practices to meet environmental regulations and reduce operational costs. This focus not only ensures compliance but also appeals to eco-conscious stakeholders and consumers.
The Functional Service Providers (FSP) market is segmented based on service type, application, and end-user. Key service types include clinical trial management, data management and biostatistics, regulatory affairs, and pharmacovigilance. Applications span various therapeutic areas such as oncology, neurology, and infectious diseases. End-users primarily consist of pharmaceutical companies, biotechnology firms, contract research organizations (CROs), and academic research institutions. This segmentation allows for targeted strategies catering to the specific needs and challenges of each category.
Clinical monitoring is the leading service type in the FSP market, accounting for over 27% of the market share. This dominance is attributed to the critical role clinical monitoring plays in ensuring the safety and efficacy of clinical trials. Data management and biostatistics services are also significant, providing essential support in handling and analyzing complex clinical data. Regulatory affairs services ensure compliance with global standards, facilitating the approval process for new therapies. Pharmacovigilance services monitor the safety of pharmaceutical products, identifying and evaluating adverse effects. Other niche services contribute to the remaining market share, addressing specialized needs within the industry.
Clinical trial management is the most prominent application area, driven by the increasing number of clinical trials and the need for efficient management of these complex processes. Data management and biostatistics applications are growing rapidly, reflecting the surge in data generated from clinical studies and the necessity for robust analysis. Regulatory affairs applications are essential for navigating the complex regulatory landscapes across different regions. Pharmacovigilance applications are expanding as safety monitoring becomes more critical in the post-market phase. In 2024, more than 38% of enterprises globally reported piloting Functional Service Providers (FSP) systems for customer experience platforms.
Pharmaceutical companies are the largest end-users of FSP services, leveraging these providers to enhance efficiency and focus on core competencies. Biotechnology firms are increasingly adopting FSP models to accelerate innovation and manage specialized tasks. Contract Research Organizations (CROs) utilize FSPs to extend their service offerings and manage resource demands. Academic research institutions engage FSPs to access specialized expertise and support complex research projects. In 2024, over 60% of Gen Z consumers showed higher trust in brands that integrate AI-powered multimodal chatbots for support.
North America accounted for the largest market share at 45% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 9.2% between 2025 and 2032.
North America leads due to robust healthcare infrastructure, high R&D investments, and early adoption of advanced technologies, with over 3,200 clinical trials conducted in 2024. Asia-Pacific’s rapid growth is fueled by expanding healthcare demands, increasing clinical trial activities, and growing outsourcing trends, particularly in China, India, and Japan, collectively accounting for more than 60% of the regional FSP volume.
What factors contribute to the dominance of this region in the FSP market?
North America holds approximately 45% of the global FSP market, driven by strong healthcare and pharmaceutical sectors. Key industries such as biotechnology, pharmaceuticals, and clinical research heavily utilize FSP services. Regulatory frameworks, including FDA guidance, facilitate streamlined clinical trials and data compliance. Technological advancements, including AI-driven analytics and digital clinical platforms, are widely adopted. Local players like Parexel International Corporation are expanding services in clinical monitoring and regulatory affairs. Consumer behavior reflects higher enterprise adoption in healthcare and finance, with over 70% of organizations outsourcing specialized functions to enhance efficiency.
How do regulatory frameworks and technological advancements influence FSP adoption in this region?
Europe represents 28% of the FSP market, led by Germany, the UK, and France. Stringent regulatory standards such as GDPR drive demand for compliant and secure service providers. Sustainability initiatives and digital transformation, including blockchain-based data integrity and AI analytics, are gaining traction. Local companies like Covance Inc. are enhancing offerings in clinical monitoring and regulatory support. Consumer behavior shows strong trust in providers that ensure compliance and data security, with 65% of enterprises integrating advanced FSP solutions in clinical operations.
What drives the rapid expansion of the FSP market in this region?
Asia-Pacific accounts for 18% of the FSP market, with China, India, and Japan as top-consuming countries. Growing clinical trial activities, cost-effective labor, and government incentives drive adoption. Advanced infrastructure and innovation hubs in cities like Bengaluru and Shanghai support technological integration, including digital trial management and AI-driven data analysis. Local players collaborate with global firms to expand service offerings. Consumer trends show over 60% of healthcare enterprises adopting outsourced FSP solutions for operational efficiency and cost reduction.
What are the key factors influencing the FSP market in South America?
South America contributes around 6% of the FSP market, led by Brazil and Argentina. Expansion of healthcare infrastructure and participation in global clinical trials drive growth. Government incentives, including tax benefits for pharmaceutical investments, encourage market development. The region is embracing digital health trends like telemedicine and electronic health records. Local players are focusing on clinical monitoring and regulatory affairs. Consumer behavior indicates increasing reliance on FSPs for efficiency, with more than 50% of enterprises outsourcing specialized services.
How do regional dynamics affect the FSP market in the Middle East and Africa?
The Middle East & Africa account for roughly 3% of the FSP market. Growth is driven by healthcare infrastructure expansion in South Africa and UAE and the oil and gas sector’s demand for specialized services. Technological modernization, including telemedicine and electronic health record systems, is accelerating adoption. Local companies are developing services in regulatory affairs and clinical trial management. Consumer behavior trends indicate increasing trust in providers offering tailored, compliant solutions, with 40% of enterprises outsourcing key functional services for operational efficiency.
United States | Market Share: 42% | Dominance driven by high R&D investment, robust clinical trial infrastructure, and early adoption of advanced technologies.
Germany | Market Share: 15% | Leadership supported by stringent regulatory standards, advanced healthcare systems, and a strong focus on compliance and data security.
The Functional Service Providers (FSP) market is characterized by a fragmented competitive environment, with over 100 active global players. The top five companies together account for approximately 35% of the total market share, reflecting a diverse and highly competitive landscape. Key strategic initiatives such as mergers, acquisitions, and partnerships are shaping the market dynamics, enabling companies to expand service portfolios and enter new geographies. Innovation is a significant differentiator, with firms investing heavily in digital transformation, including AI-driven clinical trial management, machine learning for data analytics, and advanced regulatory compliance solutions. These innovations enhance operational efficiency, reduce time-to-market for clinical trials, and improve data accuracy, intensifying competition across the market. Additionally, companies are focusing on specialized service offerings for niche therapeutic areas, further driving differentiation. The market’s fragmented nature encourages strategic collaborations and targeted investments, ensuring that both established players and emerging entrants actively compete to capture market share.
PRA Health Sciences
Covance Inc.
Charles River Laboratories International Inc.
Syneos Health
Pharmaceutical Product Development LLC (PPD)
Medpace Holdings Inc.
LabCorp
Wuxi AppTec
Accenture
Cognizant
Quanticate
BioPoint Inc.
The Functional Service Providers (FSP) market is undergoing a significant technological transformation, driven by advancements that enhance operational efficiency and service delivery. One of the most impactful developments is the integration of artificial intelligence (AI) and machine learning (ML) into clinical trial processes. These technologies enable predictive analytics for patient recruitment, real-time monitoring of trial progress, and automated data analysis, leading to reduced timelines and improved accuracy in clinical studies. Cloud computing has also become a cornerstone of FSP operations. By leveraging cloud-based platforms, FSPs can offer scalable and flexible solutions that facilitate data sharing and collaboration across global teams. This infrastructure supports the management of large datasets generated during clinical trials and ensures compliance with regulatory standards.
Furthermore, the adoption of blockchain technology is gaining momentum within the FSP sector. Blockchain's ability to provide secure, transparent, and immutable records is particularly valuable in clinical trials, where data integrity and traceability are paramount. This technology helps in maintaining accurate records of patient consent, data provenance, and audit trails, thereby enhancing trust and compliance.
The Internet of Things (IoT) is another emerging trend influencing the FSP market. IoT devices, such as wearable sensors, are being utilized to collect real-time health data from patients, offering insights into treatment efficacy and patient well-being. This continuous data stream supports personalized medicine approaches and remote patient monitoring, aligning with the industry's shift towards more patient-centric care models. Collectively, these technological advancements are reshaping the FSP landscape, enabling providers to deliver more efficient, secure, and personalized services to their clients.
In June 2024, LAPP introduced the ETHERLINE® FD bioP Cat.5e, its first bio-based Ethernet cable produced in series. This sustainable variant features a bio-based outer sheath composed of 43% renewable raw materials, reducing the carbon footprint by 24% compared to traditional fossil-based TPU sheaths. Source: www.lapp.com
In May 2024, Fortrea reported an impressive 94% on-time rate for delivering clinical trial databases into production, a critical milestone for any study. This commitment to quality is often reflective of a strong internal culture. Source: www.fortrea.com
In April 2024, PPD conducted a survey revealing that nearly nine out of ten biopharma companies now utilize Functional Service Provider (FSP) or hybrid FSP frameworks for their clinical development needs. This near-universal adoption underscores the model's effectiveness in addressing industry challenges. Source: www.ppd.com
In March 2024, Catalyst Clinical Research announced that it had executed over 1,100 FSP projects, highlighting the increasing reliance on specialized outsourcing models in the clinical research sector. Source: www.catalystcr.com
The Functional Service Providers (FSP) Market Report offers a comprehensive analysis of the global outsourcing landscape within the pharmaceutical and clinical research industries. It delves into various service types, including clinical trial design and monitoring, data management, biostatistics, regulatory affairs, and pharmacovigilance, providing insights into their adoption and impact on operational efficiency. Geographically, the report covers key regions such as North America, Europe, Asia-Pacific, South America, and the Middle East & Africa, highlighting regional market dynamics, growth drivers, and challenges. It also examines the adoption of emerging technologies like artificial intelligence, machine learning, cloud computing, and blockchain, assessing their influence on service delivery and compliance standards.
The report further explores industry-specific applications, focusing on biopharmaceutical companies, medical device manufacturers, and contract research organizations. It provides data on outsourcing trends, cost optimization strategies, and the shifting focus towards core competencies. Additionally, the report addresses regulatory considerations, including compliance with global standards and the impact of data privacy laws on outsourcing practices. It offers strategic recommendations for stakeholders to navigate the evolving FSP landscape, emphasizing the importance of technological integration, scalability, and specialized expertise in achieving competitive advantage.
| Report Attribute/Metric | Report Details |
|---|---|
|
Market Revenue in 2024 |
USD 629.08 Million |
|
Market Revenue in 2032 |
USD 987.62 Million |
|
CAGR (2025 - 2032) |
5.8% |
|
Base Year |
2024 |
|
Forecast Period |
2025 - 2032 |
|
Historic Period |
2020 - 2024 |
|
Segments Covered |
By Types
By Application
By End-User
|
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Region Covered |
North America, Europe, Asia-Pacific, South America, Middle East, Africa |
|
Key Players Analyzed |
IQVIA, Parexel International Corporation, ICON plc, PRA Health Sciences, Covance Inc., Charles River Laboratories International Inc., Syneos Health, Pharmaceutical Product Development LLC (PPD), Medpace Holdings Inc., LabCorp, Wuxi AppTec, Accenture, Cognizant, Quanticate, BioPoint Inc. |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
