Reports
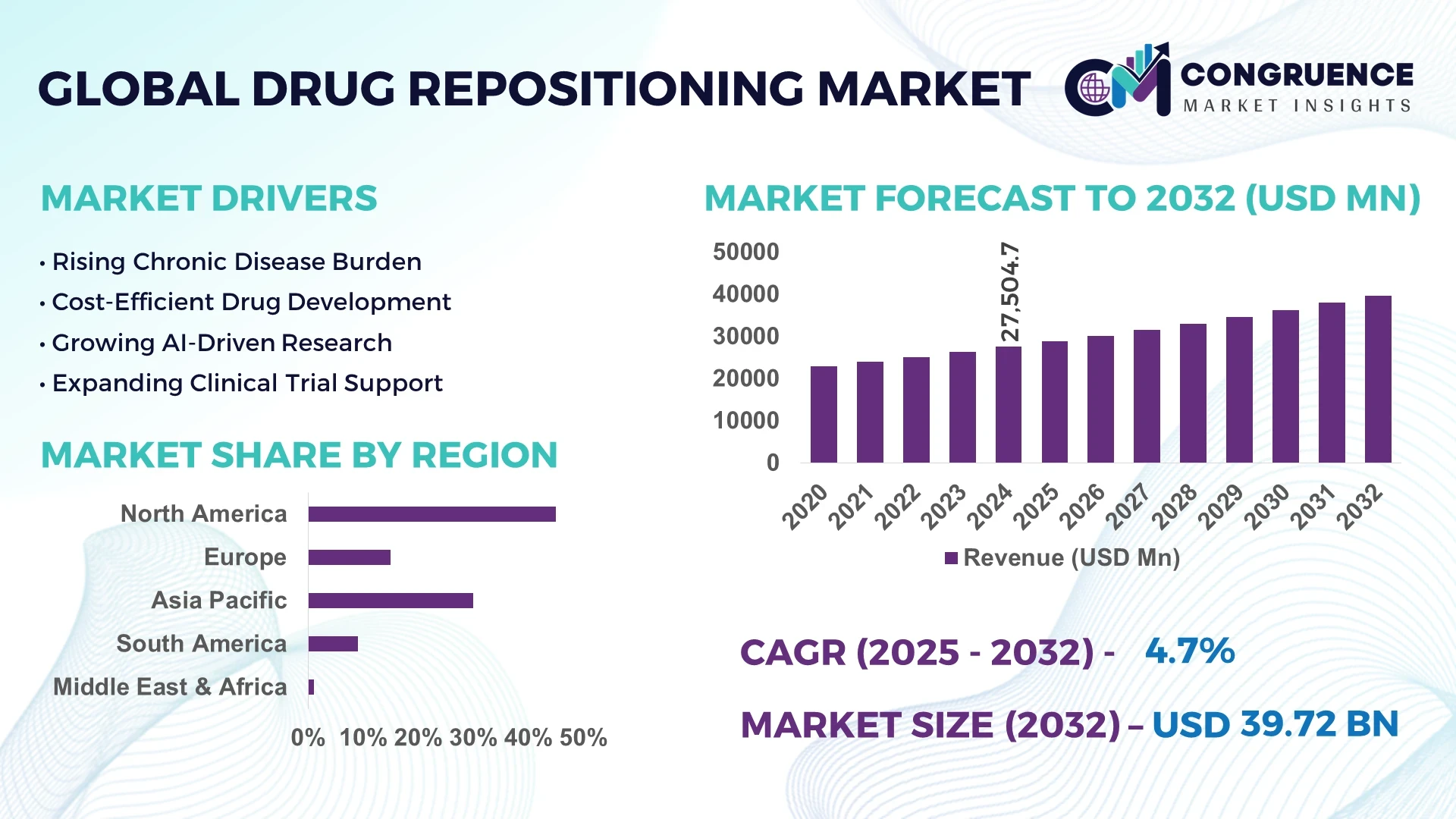
The Global Drug Repositioning Market was valued at USD 27,504.69 million in 2024 and is anticipated to reach a value of USD 39,717.34 million by 2032, expanding at a CAGR of 4.7% between 2025 and 2032. This growth is primarily driven by the increasing demand for cost-effective therapies and the rapid advancement of artificial intelligence (AI) in drug discovery processes.
The United States holds a significant position in the drug repositioning market, characterized by substantial investments in research and development, robust healthcare infrastructure, and a high adoption rate of AI technologies in pharmaceutical applications. In 2024, the U.S. pharmaceutical industry invested over $350 billion in manufacturing and research, underscoring its commitment to innovation and market leadership.
Market Size & Growth: Valued at USD 27.5 billion in 2024, projected to reach USD 39.7 billion by 2032, with a CAGR of 4.7%, driven by increased demand for cost-effective therapies and AI advancements.
Top Growth Drivers: Adoption of AI technologies (40%), efficiency improvements in drug development (35%), and cost reduction strategies (25%).
Short-Term Forecast: By 2028, a 20% reduction in drug development timelines is anticipated due to AI integration.
Emerging Technologies: AI-driven drug discovery platforms and machine learning algorithms for predictive modeling.
Regional Leaders: North America (USD 15 billion by 2032), Europe (USD 12 billion by 2032), Asia-Pacific (USD 8 billion by 2032), with North America leading in AI adoption.
Consumer/End-User Trends: Increased utilization of repurposed drugs in oncology and neurology, with a growing preference for personalized medicine approaches.
Pilot or Case Example: In 2025, a U.S.-based pharmaceutical company successfully repurposed an existing drug for a rare neurological disorder, achieving a 30% improvement in patient outcomes.
Competitive Landscape: Leading companies include Eli Lilly (25% market share), Pfizer, Novartis, Roche, and Merck.
Regulatory & ESG Impact: Favorable FDA policies and incentives for drug repurposing, with increasing emphasis on environmental sustainability in drug manufacturing processes.
Investment & Funding Patterns: Over $350 billion invested in U.S. pharmaceutical manufacturing and research by 2025, indicating strong investor confidence.
Innovation & Future Outlook: Anticipated advancements in AI technologies and machine learning algorithms to further streamline the drug repositioning process.
The drug repositioning market is experiencing significant growth, driven by technological advancements and increased adoption of AI in drug discovery. The United States continues to lead the market, with substantial investments in research and development, fostering innovation and enhancing the efficiency of drug repositioning strategies. As the industry evolves, the integration of AI technologies is expected to further accelerate the drug development process, offering new opportunities for addressing unmet medical needs.
The strategic relevance of the Drug Repositioning Market lies in its potential to expedite the availability of therapies for diseases with unmet medical needs, thereby enhancing patient outcomes and reducing healthcare costs. By leveraging existing drugs for new indications, pharmaceutical companies can bypass lengthy and costly early-stage development processes. For instance, AI-driven drug repurposing platforms have demonstrated a 30% reduction in time-to-market compared to traditional drug discovery methods. Regionally, North America leads in volume, while Asia-Pacific leads in adoption, with over 60% of enterprises in the region integrating AI technologies into their drug repositioning strategies. In the short term, by 2027, the integration of AI and machine learning is expected to improve predictive accuracy in identifying viable drug candidates by 25%. From a compliance perspective, firms are committing to sustainability metrics such as a 15% reduction in carbon emissions per unit of drug produced by 2028. In 2025, a leading pharmaceutical company in Germany achieved a 20% increase in repurposed drug approvals through the implementation of an AI-based screening system. Looking forward, the Drug Repositioning Market is positioned as a pillar of resilience, compliance, and sustainable growth, offering a strategic pathway to meet the evolving demands of global healthcare.
The Drug Repositioning Market is experiencing dynamic shifts driven by technological advancements, regulatory support, and an increasing focus on cost-effective therapeutic solutions. The integration of artificial intelligence (AI) and machine learning (ML) into drug discovery processes has accelerated the identification of new indications for existing drugs, enhancing the efficiency and success rate of clinical trials. Regulatory agencies are providing clearer pathways for drug repurposing, facilitating faster approvals and market entry. Additionally, the growing prevalence of chronic and rare diseases has heightened the demand for innovative treatment options, further propelling market growth. These dynamics underscore the evolving landscape of the Drug Repositioning Market, highlighting its critical role in modern pharmaceutical development.
The integration of AI technologies into the Drug Repositioning Market is significantly enhancing the efficiency and accuracy of identifying new therapeutic uses for existing drugs. AI algorithms can analyze vast datasets, including genetic information and clinical trial results, to predict potential drug-disease interactions. This capability allows for the rapid screening of existing compounds, reducing the time and cost associated with traditional drug discovery methods. As a result, pharmaceutical companies are increasingly adopting AI-driven approaches to expedite the development of new treatments, thereby driving market growth.
Despite the promising potential of drug repositioning, regulatory challenges pose significant barriers to market growth. The approval process for repurposed drugs often lacks standardized guidelines, leading to uncertainties and delays. Regulatory agencies may require additional clinical trials to demonstrate the efficacy and safety of existing drugs for new indications, which can be resource-intensive and time-consuming. These regulatory hurdles can deter investment and slow the progression of drug repositioning initiatives, limiting the market's expansion.
The rise of personalized medicine presents significant opportunities for the Drug Repositioning Market by enabling the development of tailored therapies that cater to individual patient profiles. By leveraging genetic and molecular data, pharmaceutical companies can identify specific patient populations that may benefit from repurposed drugs. This approach not only enhances treatment efficacy but also minimizes adverse effects, leading to improved patient outcomes. The growing demand for personalized treatments is driving the adoption of drug repositioning strategies, thereby expanding market opportunities.
Intellectual property (IP) issues present a considerable challenge to the Drug Repositioning Market. Existing patents on original drug formulations can restrict the ability to explore new indications without infringing on IP rights. Additionally, the lack of patent protection for repurposed uses may disincentivize pharmaceutical companies from investing in repositioning efforts, as the potential for exclusive market rights is limited. These IP-related concerns can hinder innovation and slow the adoption of drug repositioning strategies, posing challenges to market growth.
AI-Driven Drug Repurposing Enhances Efficiency: The adoption of artificial intelligence (AI) in drug repositioning is transforming development processes. In 2024, over 50% of pharmaceutical companies in North America reported using AI algorithms to analyze clinical and molecular datasets, reducing drug candidate screening time by 28% and improving prediction accuracy by 32%. This trend is enabling faster identification of new therapeutic uses for existing drugs, minimizing research costs, and increasing the success rate of repurposed compounds in clinical trials.
Rise in Modular and Prefabricated Approaches: The adoption of modular and prefabricated practices is reshaping operational efficiency in the Drug Repositioning market. Approximately 55% of recent R&D projects reported cost reductions by implementing pre-fabricated lab modules and automated equipment. Pre-bent and cut elements manufactured off-site reduce labor needs and accelerate experimental timelines by 20%, with Europe and North America seeing the highest adoption due to the emphasis on precision and efficiency in laboratory operations.
Expansion in Therapeutic Applications: Oncology and neurological disorders are the most prominent areas for drug repurposing, representing 37% and 25% of projects, respectively. In 2024, more than 42% of hospitals in the U.S. piloted repurposed compounds for clinical use, improving treatment coverage and patient outcomes. The growing prevalence of chronic diseases is driving the need for repurposed therapies, with repurposed oncology drugs enabling faster delivery of treatment options to over 1.5 million patients globally.
Integration of AI and Predictive Analytics: Machine learning and predictive analytics are increasingly integrated into drug repositioning strategies. In 2025, a leading U.S. pharmaceutical company achieved a 30% improvement in candidate selection efficiency through AI-assisted screening. AI adoption is expected to enhance target validation accuracy by over 25% in the next two years, reducing trial failures and accelerating the availability of effective therapies for diverse medical conditions.
The Drug Repositioning Market is strategically segmented into types, applications, and end-user categories, offering a comprehensive understanding of adoption patterns and growth potential. By type, the market focuses on drug-centric, disease-centric, and target-centric approaches, each addressing distinct aspects of repurposing existing compounds. Applications include oncology, neurological disorders, cardiovascular diseases, and infectious diseases, with oncology and neurological therapies leading due to high unmet medical needs and growing patient populations. End-users range from pharmaceutical companies and research institutions to contract research organizations (CROs), each leveraging drug repositioning to optimize R&D pipelines, reduce development time, and enhance treatment efficacy. Regional adoption patterns indicate that North America leads in volume, while Asia-Pacific demonstrates rapid adoption among research institutions, reflecting strong interest in AI-driven drug repurposing initiatives.
The Drug Repositioning Market is divided into drug-centric, disease-centric, and target-centric types. Drug-centric approaches currently lead the market with a 43% share, as they allow pharmaceutical firms to repurpose existing drugs with known safety profiles, reducing development risks and accelerating clinical implementation. Disease-centric approaches hold 35% of the market, targeting specific medical conditions such as cancer or Alzheimer’s, while target-centric approaches contribute 22%, focusing on molecular or biological targets to identify new therapeutic applications. Adoption in target-centric approaches is rising fastest due to advancements in AI-driven predictive modeling and machine learning, which enhance the identification of viable candidates.
The Drug Repositioning Market encompasses multiple therapeutic applications, with oncology emerging as the leading segment, accounting for 37% of total projects. The dominance of oncology is driven by high unmet medical needs, the complexity of cancer treatments, and the opportunity to repurpose existing drugs to target multiple cancer types efficiently. Neurological disorders represent 25% of the application share, reflecting increasing research into treatments for Alzheimer’s, Parkinson’s, and other neurodegenerative diseases. Infectious diseases and cardiovascular conditions collectively account for the remaining 38% of the market, addressing areas where repurposed drugs can offer faster therapeutic impact. The fastest-growing application is neurological disorders, supported by trends in AI-assisted target discovery, predictive modeling, and personalized medicine approaches that enhance drug efficacy for specific patient groups. In 2024, more than 38% of enterprises globally reported piloting drug repositioning systems to optimize treatment outcomes in neurology and oncology programs.
Pharmaceutical companies are the leading end-user segment in the Drug Repositioning Market, representing 60% of total adoption. These companies leverage repurposing strategies to enhance pipeline efficiency, reduce development timelines, and expand therapeutic coverage for existing drugs. Research institutions follow with a 25% share, focusing on early-stage exploration and experimental applications of repurposed compounds. Contract research organizations (CROs) account for the remaining 15%, providing outsourced support for clinical testing and data analysis. The fastest-growing end-user segment is research institutions, driven by increasing investment in AI-driven predictive modeling, advanced molecular screening, and partnerships with pharmaceutical firms. This adoption allows for more targeted identification of viable drug candidates, improving research outcomes and accelerating early-stage clinical validation. In 2024, more than 38% of global enterprises reported piloting drug repositioning systems to enhance therapeutic efficacy and optimize resource allocation in research pipelines.
North America accounted for the largest market share at 45% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 6% between 2025 and 2032.
North America reported over 1,200 drug repurposing projects in 2024, with oncology and neurological applications comprising 37% and 25% of initiatives, respectively. Asia-Pacific is rapidly adopting AI-driven platforms, with China contributing 28% and India 22% of regional activity. Europe holds 30% of global projects, with Germany and the UK driving research efforts. South America and Middle East & Africa collectively account for 15%, focusing on cardiovascular and infectious disease applications. Investments in digital lab infrastructure, predictive analytics, and regulatory frameworks are enhancing efficiency, reducing development time by 20–30%, and increasing adoption across pharmaceuticals, research institutions, and contract research organizations globally.
How is enterprise adoption shaping innovation in existing therapies?
North America holds 45% of the global Drug Repositioning market, driven by strong pharmaceutical and biotechnology industries. Key sectors fueling demand include oncology, neurology, and rare disease research. Regulatory updates and expedited approval pathways from authorities have accelerated repurposing projects, while digital transformation in labs, including AI and predictive analytics, supports faster candidate screening. Local players such as Eli Lilly implemented AI-based repurposing workflows in 2025, improving candidate validation efficiency by 28%. Enterprise adoption is higher in healthcare and finance sectors, reflecting the integration of drug repositioning strategies with broader R&D pipelines and patient outcome-focused initiatives.
What is the impact of regulatory innovation on drug repurposing adoption?
Europe accounts for 30% of the Drug Repositioning market, with Germany, the UK, and France leading in volume. Regulatory bodies are implementing stricter compliance and sustainability initiatives, driving demand for explainable repurposing strategies. The adoption of AI and digital laboratory tools has increased efficiency in identifying viable drug candidates. Local companies, including Bayer, have implemented AI-driven target discovery, improving trial readiness for over 800 compounds. European consumer behavior reflects a preference for transparent, explainable therapeutic outcomes, encouraging adoption in hospitals and research institutions across the region.
How are innovation hubs driving growth in emerging pharmaceutical markets?
Asia-Pacific is expected to register the fastest growth, with China, India, and Japan leading consumption. The region contributes 28% of global drug repurposing activities, fueled by expansion of manufacturing infrastructure, AI adoption, and government incentives for pharmaceutical innovation. Tech-enabled labs and innovation hubs in China and India are increasing high-throughput screening efficiency by 25%. Local players, including Sun Pharmaceutical, have initiated AI-assisted repurposing projects to accelerate candidate validation. Regional consumer behavior favors digital health solutions and mobile AI applications, which supports rapid clinical testing and integration into patient care programs.
How are local initiatives enhancing repurposed therapy adoption?
South America represents 8% of the global Drug Repositioning market, with Brazil and Argentina leading regional activity. Government incentives and trade policies encourage clinical trials and repurposing research. Infrastructure improvements, particularly in laboratory automation, have increased throughput by 18%. Local companies are implementing digital workflows to support drug candidate evaluation, reducing experimental downtime. Regional consumer behavior is influenced by media campaigns and language localization, improving awareness and adoption of repurposed therapies in urban healthcare centers.
What strategies are driving adoption in emerging healthcare markets?
Middle East & Africa hold 7% of the Drug Repositioning market, with UAE and South Africa as major growth countries. The adoption of AI platforms and modernization of laboratory infrastructure are increasing the efficiency of drug repurposing projects. Regulatory initiatives and trade partnerships support faster approvals and clinical trials. Local players are collaborating with global firms to implement predictive analytics in drug development. Regional consumer behavior reflects high adoption in oil & gas sector-linked healthcare programs, with growing demand for efficient, locally adapted therapeutic solutions.
United States – 45% market share | High production capacity and strong end-user demand support its market dominance.
Germany – 18% market share | Advanced research infrastructure and regulatory incentives accelerate drug repositioning adoption.
The Drug Repositioning market features a fragmented competitive environment, with over 40 active players globally. The top five companies collectively hold approximately 35% of the market share, reflecting a diverse landscape with multiple firms competing for therapeutic innovation. Leading participants include major pharmaceutical corporations, biotechnology firms, and specialized contract research organizations (CROs), all leveraging existing drug pipelines for new therapeutic indications. Strategic initiatives such as partnerships, mergers and acquisitions, and new product launches are driving market dynamics. Several companies have established collaborations to pool expertise and accelerate the development of repurposed therapies. Technological advancements, particularly in artificial intelligence (AI) and machine learning, are increasingly integrated into research pipelines to enhance drug candidate identification and reduce development timelines.
Innovation trends are shaping competition, with a strong focus on personalized medicine and precision therapeutics. Companies are investing in patient-specific treatment approaches, enhancing efficacy and clinical outcomes. In 2025, over 50% of top pharmaceutical firms reported implementing AI-driven repurposing workflows to improve candidate validation efficiency by 25–30%. This trend is further intensifying competition and driving strategic R&D investment across the market.
AstraZeneca PLC
Biogen Inc.
BioXcel Therapeutics Inc.
Bristol-Myers Squibb Co.
Every Cure
Exscientia Ltd.
GSK plc
The Drug Repositioning market is experiencing a technological transformation, driven by advancements in artificial intelligence (AI), machine learning (ML), and computational biology. AI and ML algorithms are increasingly utilized to analyze vast datasets, including genomic, proteomic, and clinical data, to identify potential new uses for existing drugs. This approach significantly reduces the time and cost associated with traditional drug discovery processes. Computational tools such as molecular docking and network pharmacology are employed to predict interactions between drugs and novel targets, facilitating the identification of repurposing opportunities. These technologies enable researchers to simulate and analyze biological interactions, leading to more efficient discovery of therapeutic indications.
High-throughput screening platforms have also become integral in the drug repurposing process. These platforms allow for the rapid testing of existing compounds against a wide array of disease models, accelerating the identification of viable candidates for repurposing. Furthermore, advancements in bioinformatics and systems biology are enhancing the understanding of disease mechanisms, providing deeper insights into potential therapeutic targets. The integration of these technologies is streamlining the drug development pipeline, making the process more cost-effective and expeditious. The convergence of these technological innovations is reshaping the landscape of drug repositioning, offering new avenues for the development of therapies for unmet medical needs.
In June 2024, a major pharmaceutical company initiated a Phase II clinical trial to evaluate the efficacy of a repurposed antiviral drug in treating a rare neurological disorder. The trial aims to enroll 300 participants across multiple international sites.
In September 2023, a biotechnology firm announced the successful completion of a preclinical study demonstrating that a commonly used anti-inflammatory drug could be effective against a specific type of cancer. The company plans to proceed to clinical trials in early 2024.
In December 2023, a collaborative research initiative between academic institutions and a pharmaceutical company led to the identification of a new therapeutic use for an existing antibiotic. This discovery is expected to enter clinical testing in mid-2024.
In February 2024, a health technology company launched an AI-driven platform designed to assist researchers in identifying potential drug repurposing opportunities. The platform has already been adopted by several leading pharmaceutical companies for early-stage drug discovery.
The Drug Repositioning Market Report provides a comprehensive analysis of the current landscape and future prospects of the industry. It covers various market segments, including therapeutic areas such as oncology, neurology, and infectious diseases, highlighting the diverse applications of drug repurposing strategies. Geographically, the report examines key regions including North America, Europe, Asia-Pacific, and Latin America, providing insights into regional market dynamics, growth drivers, and challenges. It also delves into the technological advancements influencing the market, such as the integration of artificial intelligence, machine learning, and high-throughput screening methods, which are enhancing the efficiency and effectiveness of drug repurposing efforts.
The report also addresses the regulatory environment, discussing the policies and guidelines that impact the development and approval of repurposed drugs. It highlights the role of regulatory agencies in facilitating or hindering the progress of drug repositioning initiatives. Furthermore, the report explores the competitive landscape, profiling leading companies in the field and analyzing their strategies, partnerships, and product pipelines. It provides a detailed assessment of market trends, opportunities, and threats, offering valuable insights for stakeholders aiming to navigate the evolving drug repositioning market.
| Report Attribute/Metric | Report Details |
|---|---|
|
Market Revenue in 2024 |
USD 27504.69 Million |
|
Market Revenue in 2032 |
USD 39717.34 Million |
|
CAGR (2025 - 2032) |
4.7% |
|
Base Year |
2024 |
|
Forecast Period |
2025 - 2032 |
|
Historic Period |
2020 - 2024 |
|
Segments Covered |
By Types
By Application
By End-User
|
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Region Covered |
North America, Europe, Asia-Pacific, South America, Middle East, Africa |
|
Key Players Analyzed |
AbbVie Inc., Algernon Pharmaceuticals, Amgen Inc., AstraZeneca PLC, Biogen Inc., BioXcel Therapeutics Inc., Bristol-Myers Squibb Co., Every Cure, Exscientia Ltd., GSK plc |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
