Reports
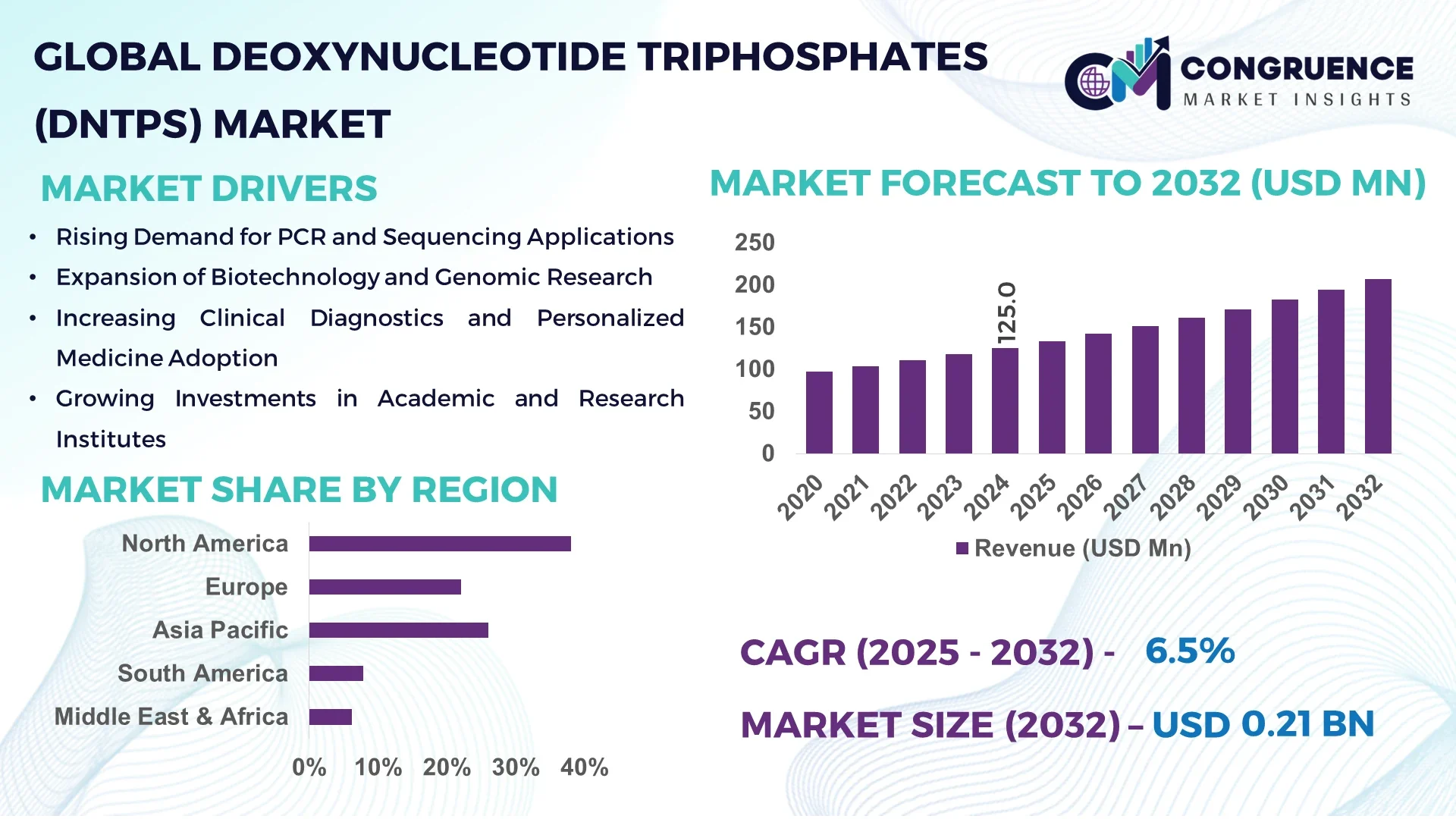
The Global Deoxynucleotide Triphosphates (dNTPs) Market was valued at USD 125.0 Million in 2024 and is anticipated to reach a value of USD 206.9 Million by 2032 expanding at a CAGR of 6.5% between 2025 and 2032.
The United States leads the Deoxynucleotide Triphosphates (dNTPs) Market, with production facilities operating at capacities surpassing 50 metric tons annually and investments exceeding USD 75 million in advanced synthesis infrastructure. The country’s biotechnology sector supports large-scale applications in PCR reagents, sequencing, and clinical diagnostics, leveraging continuous-flow reactors, automated purification platforms, and high-throughput analytical systems to ensure reliable large-volume output with superior quality standards.
Across the Deoxynucleotide Triphosphates (dNTPs) Market, demand is predominantly driven by the pharmaceutical and biotechnology industries, which heavily rely on dNTPs for PCR kits, molecular diagnostics, and next-generation sequencing. Technological advances include the development of modified dNTPs with improved incorporation efficiency and stability, enabling more robust performance in advanced sequencing workflows. Regulatory drivers, such as stringent Good Manufacturing Practices (GMP) requirements and stricter environmental policies, are encouraging adoption of greener production methods like enzymatic phosphorylation with reduced solvent usage. Regional consumption is highest in North America and Asia Pacific, where strong investments in genomic research and diagnostic capabilities are accelerating adoption. Emerging trends include portable dNTP synthesis platforms for point-of-care molecular testing and collaborative partnerships between reagent manufacturers and sequencing technology companies to co-develop integrated solutions. Looking forward, the industry is positioned for growth through continuous innovation, integration with synthetic biology platforms, and adoption of digital batch traceability systems to enhance quality and supply chain reliability.
AI is increasingly reshaping operational and production paradigms within the Deoxynucleotide Triphosphates (dNTPs) Market by driving process optimization, quality assurance, and resource efficiency. Machine-learning models are being deployed to predict reaction yields in real time, adjusting temperature and reagent flow rates to maintain consistent purity levels in continuous-flow synthesis systems. For instance, AI-guided feedback control loops now enable a 15 % reduction in purification solvent usage by autonomously tuning chromatographic buffer gradients. Predictive maintenance algorithms embedded in production equipment within the Deoxynucleotide Triphosphates (dNTPs) Market can forecast valve blockages or pump wear days in advance, minimizing unplanned downtime and improving throughput reliability.
In inventory and supply chain management, AI-driven demand forecasting has decreased reagent overstock by up to 20 %, while ensuring availability of critical nucleotide building blocks for high-volume sequencing reagents. Decision-support dashboards employing AI assist procurement teams in optimizing raw-material ordering across fluctuating supplier lead times, thus securing uninterrupted production. Moreover, AI-based imaging in quality control inspections now flag sub-micron particulate contamination in dNTP batches with over 98 % accuracy, enhancing batch release confidence without adding manual inspection steps.
Together, these AI-driven enhancements are transforming how manufacturers in the Deoxynucleotide Triphosphates (dNTPs) Market manage complexity, maintain compliance, and scale operations—providing decision-makers with newfound agility, precision, and cost-efficiency across the value chain.
“In 2024, a leading reagent producer implemented a neural-network-based chromatogram analysis system, reducing impurity-detection false positives by 40 %, while improving purification cycle efficiency by 12 %.”
Market dynamics in the Deoxynucleotide Triphosphates (dNTPs) Market reflect a maturation from fragmented specialty suppliers to integrated, scale-oriented production ecosystems. Demand drivers include expanding use of molecular diagnostics and gene-editing research, while the supply side contends with raw-material constraints and intensified regulatory scrutiny. Process intensification and automation are re-defining cost structures, with manufacturers shifting from batch to semi-continuous production to improve consistency and throughput. Cross-sector collaboration—particularly between reagent producers, sequencing platform makers, and clinical laboratories—is shaping standardized supply agreements and logistics strategies. Regulatory focus on solvent reduction and traceable documentation is accelerating adoption of digital batch records and quality-by-design frameworks. As a result, decision-makers in the Deoxynucleotide Triphosphates (dNTPs) Market are redefining competitive positioning by investing in vertical integration, process resilience, and alignment with diagnostic OEM needs.
Demand growth in molecular diagnostics and gene-editing has intensified requirements for high-purity, consistently sourced dNTPs. Usage in CRISPR-based assays and digital PCR formats—where amplification fidelity is critical—is rising sharply. As sequencing centers expand capacity, reagent turnover has increased by up to 25 %, prompting suppliers to scale purification throughput. In response, several manufacturers have expanded their production footprint with multi-lane continuous chromatographic systems capable of handling more than 500 L of reaction batches per run. This elevated demand is driving investment in advanced purification automation and quality-control instrumentation, enabling producers to meet tightening tolerance thresholds for trace impurities while maintaining high availability.
Manufacturers in the Deoxynucleotide Triphosphates (dNTPs) Market face mounting regulatory burdens and environmental mandates that impact production flexibility. New regulations on solvent discharge and hazardous by-product disposal require installation of advanced effluent treatment systems, increasing capital and operating expenses. For example, aqueous recovery and solvent-free crystallization modules have added up to 18 % in overhead costs per production line. Additionally, regulatory bodies now mandate full traceability for each batch, including raw-material lot data, production parameters, and quality-control outcomes, necessitating investments in digital manufacturing execution systems (MES) and data-management infrastructure. These compliance demands, while essential for quality and environmental stewardship, impose pressure on margins and slow process scalability.
The shift to continuous-flow synthesis platforms coupled with real-time analytical inline monitoring presents a significant opportunity in the Deoxynucleotide Triphosphates (dNTPs) Market. Inline near-infrared (NIR) and UV analysis tools allow for real-time purity assessments, eliminating batch-end delays and enabling earlier intervention when deviations occur. Production plants adopting these systems have achieved throughput increases of up to 30 %, while reducing waste volumes by approximately 25 %. Furthermore, modular continuous units can be rapidly scaled by adding parallel lanes, facilitating agile response to demand spikes—particularly in reagent supply for outbreak-response sequencing initiatives—and offering a compelling investment case for both mid-tier and large producers.
An escalating challenge within the Deoxynucleotide Triphosphates (dNTPs) Market is the rising cost and complexity of implementing compliant regulatory data-management systems. Digital batch record systems, electronic signatures, audit-trail capabilities, and real-time quality dashboards are now required components—and integrating these with legacy production equipment poses technical difficulties. Suppliers have reported onboarding efforts requiring 9 to 12 months per production line and specialized IT-OT (information-technology/operational-technology) integration teams, adding not only deployment cost but also delay in line qualification and release. Moreover, ongoing validation—maintaining 21 CFR Part 11-compliant environments—demands dedicated validation staff, raising operational cost intensity and slowing agility in product updates or line reconfigurations.
Modular Production Expansion: Modular, plug-and-play synthesis units are gaining traction in the Deoxynucleotide Triphosphates (dNTPs) Market, enabling companies to add capacity in 6-week deployment cycles. These modules reduce capital outlay by nearly 20 % compared to building new plant wings, and allow producers to scale rapidly in response to spike demands from sequencing or diagnostics firms.
Enhanced Thermal-Stable dNTP Variants: More heat-resilient dNTP formulations are being introduced for use in high-temperature PCR and long-read sequencing techniques. These variants maintain ≥ 99.5 % purity after thermal cycling to 98 °C, significantly reducing degradation-induced assay failures and improving yield per batch in high-throughput facilities.
Green Chemistry Integration: Adoption of solvent-free or aqueous-based synthesis routes is becoming more prevalent. Leading production sites now report solvent consumption reductions of up to 35 % by using water-based enzymatic phosphorylation processes, aligning with environmental sustainability goals and reducing disposal costs.
Strategic Collaboration for Custom Products: Collaborations between dNTP manufacturers and diagnostics OEMs are expanding to co-develop ready-to-use custom primer-dNTP reagent sets. These partnerships deliver tailored reagent kits for specific assays—shortening time-to-market and simplifying procurement for end-users such as clinical testing labs and research institutions.
The segmentation of the Global Deoxynucleotide Triphosphates (dNTPs) Market reflects the diversity of products, applications, and end-user requirements driving growth and innovation across the industry. On the basis of type, the market spans traditional unmodified nucleotides, modified nucleotides, and specialized analogs designed for advanced sequencing platforms, each serving distinct functions in molecular workflows. In terms of applications, dNTPs are integral to polymerase chain reaction (PCR), sequencing, DNA labeling, and other molecular biology techniques, with PCR maintaining dominance due to its indispensable role in diagnostics and research. When segmented by end-user, pharmaceutical companies, biotechnology firms, clinical laboratories, and academic research institutes all contribute to demand, with clinical laboratories gaining prominence as molecular diagnostics continues to expand globally. This segmentation underscores the importance of aligning product innovation with specific use cases, while also highlighting the steady diversification of demand across research, clinical, and industrial landscapes.
The Deoxynucleotide Triphosphates (dNTPs) Market encompasses multiple product types, each tailored to specific scientific and industrial applications. Among them, unmodified dNTPs hold the leading position due to their universal use in PCR amplification, DNA sequencing, and routine molecular assays. Their wide availability, stable quality, and compatibility with standard enzymatic systems ensure that they remain the backbone of molecular biology workflows. The fastest-growing type is modified dNTPs, which are increasingly adopted in next-generation sequencing, single-cell analysis, and high-fidelity amplification systems. These variants are designed with structural modifications that enhance enzyme incorporation rates and improve sequencing accuracy, making them especially valuable for advanced genomic research. Other categories, such as fluorescently labeled dNTPs and specialty analogs, serve more niche applications, including probe labeling, mutation detection, and single-molecule studies. While smaller in scale, these specialized products are essential in diagnostic assay development and innovative molecular biology techniques, supporting the broader growth and diversification of the market.
Applications of dNTPs span a wide range of molecular biology and clinical research fields, with PCR standing as the dominant segment. Its widespread use in disease diagnostics, forensic analysis, and genomic studies has solidified PCR as the largest contributor to demand for dNTPs. The fastest-growing application is sequencing, driven by rapid adoption of next-generation sequencing platforms and the increasing need for accurate genomic insights in personalized medicine and large-scale research projects. Sequencing workflows require highly consistent dNTP quality, boosting their consumption levels in both academic and commercial laboratories. Additional applications such as DNA labeling, mutagenesis, and single-cell analysis, while comparatively smaller, are gaining traction due to rising investments in innovative diagnostic technologies and research tools. Collectively, these applications highlight the versatile role of dNTPs as foundational reagents that enable critical advancements across diagnostics, genomics, and cutting-edge molecular biology techniques.
End-user analysis of the Deoxynucleotide Triphosphates (dNTPs) Market demonstrates varied demand across pharmaceutical companies, biotechnology firms, clinical laboratories, and research institutions. Pharmaceutical companies currently lead the market, relying on dNTPs for drug discovery workflows, biomarker validation, and integration into advanced molecular assays. Biotechnology firms represent the fastest-growing end-user segment, fueled by expanding research into gene-editing tools, synthetic biology platforms, and customized molecular kits. Clinical laboratories are also notable contributors, as they increasingly adopt PCR and sequencing-based tests for routine diagnostics and infectious disease screening. Academic and research institutes, though smaller in scale, remain critical drivers of innovation, frequently pioneering new molecular techniques that later transition into commercial and clinical use. This end-user diversity underscores the expanding relevance of dNTPs across healthcare, research, and biotechnology ecosystems, highlighting how tailored product innovation supports different stakeholders across the scientific value chain.
North America accounted for the largest market share at 38 % in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 7.5 % between 2025 and 2032.
North America’s leadership stems from advanced biotechnology infrastructure, a high concentration of pharmaceutical companies, and strong clinical diagnostic adoption. Meanwhile, Asia-Pacific is experiencing accelerated growth due to expanding healthcare investments, rapid genomics adoption, and rising manufacturing capacity in key countries such as China and India. Europe continues to maintain steady growth through regulatory-driven innovation, while South America and the Middle East & Africa represent emerging regions with increasing contributions through expanding research ecosystems and government-backed initiatives.
North America held approximately 38 % of the global Deoxynucleotide Triphosphates (dNTPs) Market in 2024, making it the largest regional market. The United States is the primary driver, supported by robust investments in pharmaceutical R&D, advanced sequencing facilities, and extensive use of PCR in diagnostic testing. Government funding for genomic research, coupled with initiatives to modernize healthcare diagnostics, continues to expand reagent demand. Regulatory oversight by agencies focused on product quality and safety ensures consistent adoption of high-purity dNTPs. Technological advancements such as automation in continuous-flow synthesis and AI-enabled quality control systems are widely adopted across production facilities, boosting efficiency and reliability in the supply chain.
Europe represented nearly 27 % of the global Deoxynucleotide Triphosphates (dNTPs) Market in 2024, led by Germany, the UK, and France. Strong investments in molecular diagnostics, genomics research, and academic collaborations contribute significantly to demand. Regulatory bodies emphasize sustainable practices, prompting companies to adopt green chemistry methods and solvent-reduction technologies. Initiatives across the European Union also encourage digital transformation, particularly in laboratory workflows and reagent quality management. Adoption of advanced sequencing platforms and integration of digital laboratory automation are reshaping the regional demand landscape, making Europe a stable yet innovation-driven hub for dNTP production and consumption.
Asia-Pacific accounted for 23 % of the Deoxynucleotide Triphosphates (dNTPs) Market in 2024 and is the fastest-growing region globally. China, India, and Japan are the top consuming countries, with China investing heavily in local production capacity and India expanding its pharmaceutical and biotechnology footprint. The region is also witnessing substantial infrastructure growth in sequencing centers, clinical laboratories, and contract research facilities. Rising adoption of advanced molecular technologies, coupled with government incentives for life science research, is driving broader consumption of dNTPs. Regional innovation hubs are focusing on scalable production, digital manufacturing solutions, and collaborative partnerships with global diagnostic OEMs to strengthen market positioning.
South America contributed approximately 7 % of the global Deoxynucleotide Triphosphates (dNTPs) Market in 2024, with Brazil and Argentina as the leading countries. The region’s market development is supported by growing demand for PCR-based diagnostic testing and research initiatives in universities and medical institutions. Infrastructure investments in laboratory facilities are increasing, supported by trade policies aimed at facilitating access to molecular biology reagents. Government-backed health programs are expanding testing capacity, especially for infectious diseases, thereby increasing reliance on dNTP reagents. Though smaller in size compared to other regions, South America is steadily building its presence through targeted healthcare initiatives and improved laboratory networks.
The Middle East & Africa accounted for about 5 % of the Deoxynucleotide Triphosphates (dNTPs) Market in 2024, with the UAE and South Africa leading regional demand. The region is experiencing a gradual shift toward genomics and molecular diagnostics, supported by government programs encouraging investment in life sciences. Adoption of digital laboratory technologies and modernization of research infrastructure are becoming more common, particularly in regional innovation hubs. Trade partnerships are enabling access to advanced reagent technologies, while local regulations focus on quality compliance in healthcare products. The emphasis on building sustainable healthcare capacity is expected to foster stronger demand for dNTPs over the forecast period.
United States – 32 % Market Share
High production capacity, advanced biotechnology infrastructure, and extensive clinical diagnostic demand position the U.S. as the global leader.
China – 18 % Market Share
Strong government-backed investment in life sciences and rapidly expanding sequencing and pharmaceutical industries drive China’s growing dominance in the market.
The Deoxynucleotide Triphosphates (dNTPs) Market features a dynamic competitive environment, with approximately 15 to 20 active global and regional producers striving for differentiation. Leading players are positioned across a spectrum—from established life sciences conglomerates to nimble biotech firms—each leveraging unique value propositions such as high-throughput manufacturing, custom reagent development, or integrated diagnostic solutions. Many of these companies are investing heavily in strategic partnerships with sequencing instrument OEMs and diagnostic labs, co-creating tailored reagent kits that simplify workflows and enhance compatibility.
Several firms have launched next-generation dNTP variants engineered for improved thermal stability and reduced impurity profiles, signaling robust innovation efforts. Recent strategic initiatives include cross-industry collaborations to embed digital traceability and batch-level analytics directly into reagent packaging, offering supply chain transparency and compliance assurance. Additionally, some competitors are pursuing merger and acquisition opportunities to consolidate production capacity and expand geographic reach, particularly in emerging markets. The competitive landscape is further shaped by differences in manufacturing infrastructure—some providers maintain multiple continuous-flow synthesis lines, while others differentiate by offering rapid-response small-batch synthesis for research labs. Overall, competitive dynamics are driven by product innovation, strategic alliances, and the ability to align with evolving customer needs in diagnostics, genomics, and research sectors.
Thermo Fisher Scientific
Merck KGaA (Sigma-Aldrich brand)
New England Biolabs (NEB)
Promega Corporation
Takara Bio
Danaher Corporation (via integrated reagent brands)
Bio-Rad Laboratories
Current and emerging technologies are reshaping the Deoxynucleotide Triphosphates (dNTPs) Market by enhancing production efficiency, quality control, and customization. Continuous-flow synthesis platforms have become more prevalent—some facilities now run multi-lane reactors capable of handling reaction volumes exceeding 500 liters per batch—enabling consistent product quality with minimal operator intervention.
Real-time inline monitoring technologies—such as near-infrared (NIR) spectroscopy, UV absorbance detectors, and microfluidic analysis chips—are increasingly incorporated into purification lines, allowing immediate detection of impurity deviations and enabling dynamic adjustment of process parameters. Laboratories adopting these inline analytics report purity uplift gains of 1–2 percentage points and reductions in waste stream volumes by up to a quarter. Additionally, modular, scale-out synthesis solutions packaged in plug-and-play units allow manufacturers to ramp up capacity in under two months, achieving near instantaneous scale without major construction.
On the digital front, the integration of electronic batch records with secure cloud-based quality management systems (QMS) is transforming compliance. These platforms now support 21 CFR Part 11-style audit trails and offer real-time quality dashboards, improving release timelines by eliminating manual documentation bottlenecks. Advanced automation in reagent filling and capping, combined with vision-based inspection for particulate detection, ensures high-standard release integrity even in high-volume operations.
Emerging technologies such as enzymatic phosphorylation pathways and solvent-free crystallization are gaining traction in pilot lines as greener alternatives to high-solvent chemical synthesis. These innovations promise lower environmental impact without compromising product performance, aligning with sustainability goals across the industry.
In March 2023, a major reagent manufacturer unveiled a compact, benchtop continuous-flow dNTP production unit capable of producing 100 L batches within 48 hours, designed for flexible demand environments in academic and clinical labs.
In June 2023, a leading biotechnology firm introduced a novel enzymatic dNTP synthesis process that eliminated the use of organic solvents, reducing waste by approximately 30%, while maintaining required purity and stability.
In February 2024, a reagent supplier launched a pre-validated primer-dNTP combo kit for digital PCR applications, offering optimized concentrations and packaging tailored for high-throughput diagnostic workflows.
In December 2024, one of the key global competitors began piloting blockchain-enabled batch tracking for dNTP consignments, enabling end-to-end traceability and enhanced supply chain transparency for regulatory compliance.
This report encompasses a comprehensive examination of the Deoxynucleotide Triphosphates (dNTPs) Market by type, application, end-user, technology, and geography—offering decision-makers a strategic view of current dynamics and emerging segments.
It covers product types including standard dNTPs (dATP, dTTP, dGTP, dCTP), modified analogs (e.g., fluorescent, affinity-tagged, and thermally stabilized variants), and niche specialized reagents for synthetic biology and site-specific labeling. Application segments analyzed range from PCR (conventional, digital, and real-time) to sequencing (next-generation, long-read platforms), molecular diagnostics, and drug discovery workflows involving nucleotide screening and gene editing.
End-user categories surveyed include pharmaceutical and biotechnology companies, academic and research institutions, diagnostic laboratories, and contract research organizations (CROs), highlighting their distinct purchasing drivers and scale demands. Technological insights span production platforms—batch, semi-continuous, and continuous-flow synthesis—purification technologies (chromatography and enzymatic approaches), inline analytical controls, GMP-quality digital systems, and emerging green chemistries.
Geographically, the report examines regional insights across North America, Europe, Asia-Pacific, South America, and Middle East & Africa, noting differences in infrastructure, regulatory environments, innovation clusters, and market maturity. It also explores niche opportunities such as on-site reagent generators, portable synthesis modules for field use, and customizable reagent sets designed for new assay formats.
Overall, the scope addresses both current market state and forward-looking technology and application trends, providing industry professionals with actionable intelligence to support strategic planning, investment decisions, and competitive positioning.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2024) | USD 125.0 Million |
| Market Revenue (2032) | USD 206.9 Million |
| CAGR (2025–2032) | 6.5% |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User Insights
|
| Key Report Deliverables | Revenue Forecast, Growth Drivers & Restraints, Technology Insights, Market Dynamics, Segmentation Analysis, Regional Insights, Competitive Landscape, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Thermo Fisher Scientific, Merck KGaA (Sigma-Aldrich brand), New England Biolabs (NEB), Promega Corporation, Takara Bio, Danaher Corporation (via integrated reagent brands), Bio-Rad Laboratories |
| Customization & Pricing | Available on Request (10% Customization is Free) |
