Reports
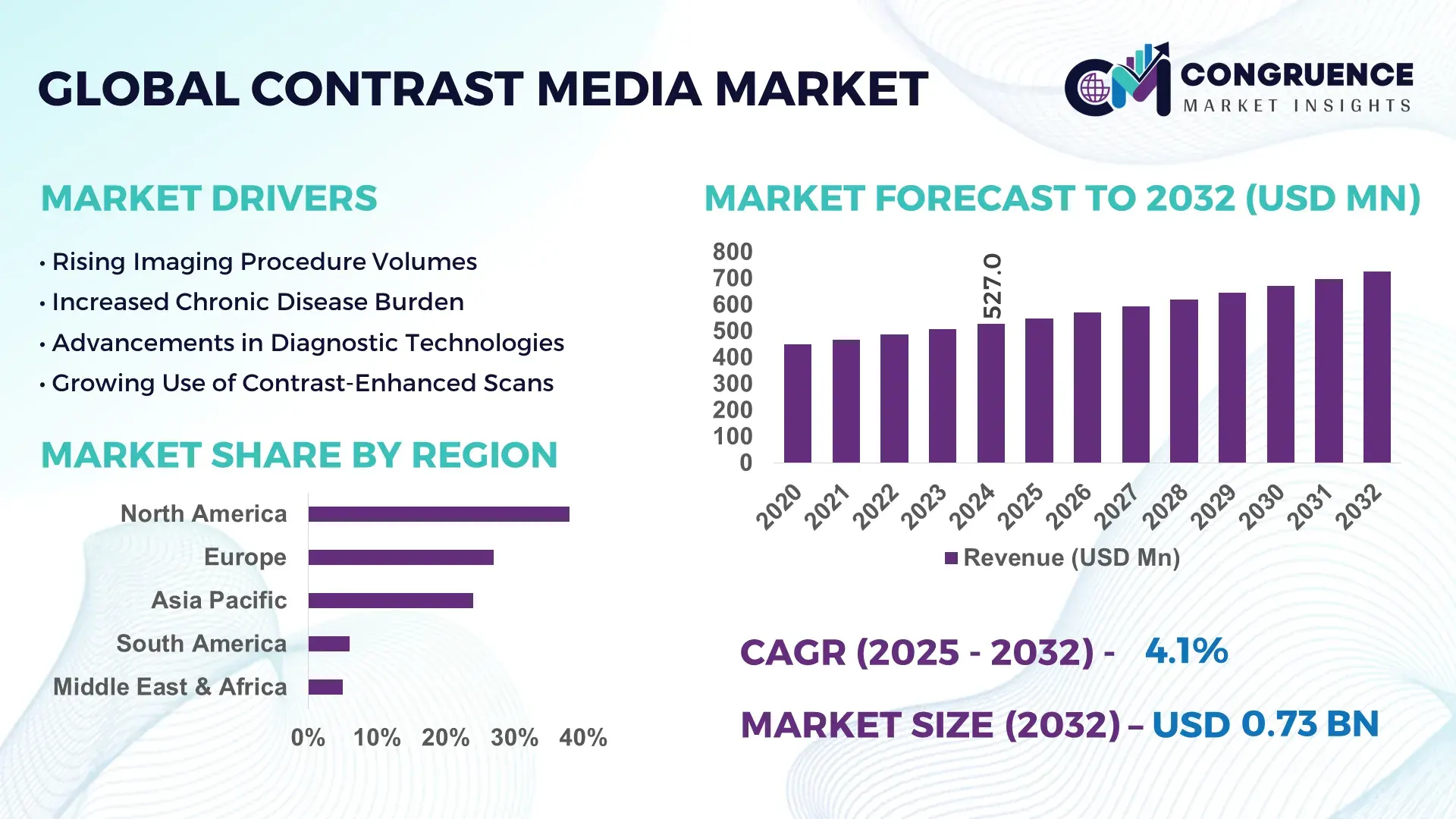
The Global Contrast Media Market was valued at USD 527.0 Million in 2024 and is anticipated to reach USD 726.8 Million by 2032, expanding at a CAGR of 4.10% between 2025 and 2032, according to an analysis by Congruence Market Insights. This growth is driven by increasing diagnostic imaging utilization and expanding radiology infrastructure worldwide.
The United States remains the leading hub for contrast media innovation, supported by advanced manufacturing capacity exceeding 290 million diagnostic doses annually, large-scale investments into next-generation contrast agent R&D surpassing USD 1.4 billion, and strong integration across MRI, CT, and interventional radiology applications. The country’s technological edge is further reinforced by early adoption of AI-enhanced imaging protocols, microbubble contrast innovations, and high regulatory emphasis on product safety—supported by over 6,000 active imaging facilities equipped for contrast-based procedures.
Market Size & Growth: Valued at USD 527.0 Million in 2024, projected to reach USD 726.8 Million by 2032 at a CAGR of 4.10%; growth driven by rising deployment of advanced imaging modalities.
Top Growth Drivers: 37% increase in diagnostic imaging adoption; 28% improvement in imaging workflow efficiencies; 22% rise in minimally invasive procedures.
Short-Term Forecast: By 2028, imaging productivity is expected to improve by 18% through automated dosing and workflow optimization.
Emerging Technologies: AI-assisted contrast dosing and nanoparticle-based contrast agents are reshaping precision imaging.
Regional Leaders: North America expected to reach USD 241 Million by 2032; Europe to reach USD 198 Million with strong radiology integration; Asia-Pacific projected at USD 175 Million driven by imaging infrastructure upgrades.
Consumer/End-User Trends: Hospitals account for the majority of usage with rising outpatient imaging demand and a strong shift toward high-accuracy diagnostic workflows.
Pilot or Case Example: In 2027, a precision-contrast dosing pilot in Japan achieved a 26% reduction in contrast waste with automated control systems.
Competitive Landscape: GE Healthcare leads with an estimated 28% share, followed by Bayer, Bracco, Guerbet, and Lantheus.
Regulatory & ESG Impact: Global regulations encourage 30% reduction in contrast waste and enhanced recycling practices by 2030.
Investment & Funding Patterns: Over USD 820 Million invested recently in imaging R&D, emphasizing sustainable and ultra-low-dose contrast technologies.
Innovation & Future Outlook: Integration of AI, microbubble agents, and hybrid imaging platforms is expected to reshape next-generation diagnostic precision.
Contrast media demand is increasingly shaped by expanding radiology capacity in emerging economies and the introduction of low-osmolality formulations optimized for patient safety. Technological advancements such as AI-driven contrast injection systems, nanoparticle-based agents, and hybrid imaging platforms are accelerating clinical adoption. Regulatory emphasis on safety, sustainable contrast usage, and workflow standardization is further driving uptake across diverse medical sectors.
The Contrast Media Market holds critical strategic importance as healthcare systems rapidly expand imaging utilization for early diagnosis, oncology staging, and minimally invasive interventions. With diagnostic imaging volumes increasing by 8–12% annually across major healthcare networks, the market is evolving toward higher-precision, lower-risk contrast formulations supported by advanced injection technologies. Comparative benchmarks indicate that AI-enabled contrast delivery systems deliver 34% improvement in dosage accuracy compared to older manual injection standards, reducing waste and enhancing diagnostic clarity.
Regional performance further highlights the market’s complexity: North America dominates in volume, while Asia-Pacific leads in adoption with over 48% of healthcare enterprises integrating enhanced contrast protocols by 2027. By 2029, AI-driven contrast optimization is expected to improve imaging turnaround times by 22%, reducing operational bottlenecks in high-volume radiology centers. ESG commitments are also reshaping the landscape, with firms targeting 30% reduction in contrast agent waste and improved recycling processes by 2030.
A practical micro-scenario illustrates this transformation: In 2028, a leading European health system achieved 19% reduction in adverse reactions through the deployment of smart injection algorithms integrated with patient risk profiles. These advancements highlight a decisive shift toward safer, higher-precision imaging ecosystems. Looking ahead, the Contrast Media Market is positioned as a pillar of diagnostic resilience, regulatory compliance, and sustainable medical growth.
The Contrast Media Market is influenced by technological innovation, expanding diagnostic imaging capacity, and increasing clinical emphasis on early disease detection. Advancements in MRI and CT imaging protocols, combined with automated injection systems, are improving workflow efficiencies and image accuracy. Growth is also supported by increasing demand for minimally invasive procedures and oncology imaging, where precision and clarity are critical. Additionally, regulatory focus on patient safety, low-osmolality formulations, and standardized imaging practices is shaping product development and market evolution globally. As healthcare providers modernize infrastructure and integrate digital tools, the market continues to benefit from heightened clinical demand and workflow optimization.
The rising adoption of advanced imaging technologies is a major driver for the Contrast Media Market, supported by substantial growth in CT and MRI utilization across healthcare systems. CT imaging volumes have increased by more than 15% globally, while MRI procedures continue to expand due to their diagnostic accuracy in neurology, cardiology, and oncology. This increased imaging activity directly elevates the need for high-quality contrast formulations, including low-osmolality iodinated agents and gadolinium-based compounds. The integration of automated injection systems, which enhance dose precision by up to 30%, supports safer and more consistent clinical workflows. Growing demand for minimally invasive and image-guided interventions further amplifies the requirement for specialized contrast media optimized for vascular, soft-tissue, and organ-level visualization. These combined technology and application advancements significantly strengthen market momentum.
Safety risks associated with contrast media, including hypersensitivity reactions and nephrotoxicity, pose ongoing challenges for market expansion. Regulatory agencies have tightened compliance standards, requiring stringent monitoring of gadolinium deposition and contrast-induced nephropathy cases, which affect an estimated 2–6% of high-risk patients. These regulatory pressures increase development costs, as manufacturers must invest in safer, more stable formulations and enhanced clinical trial requirements. Additionally, growing concern over environmental accumulation of gadolinium-based agents has prompted disposal and recycling regulations that increase operational burdens for healthcare providers. Limited accessibility to advanced contrast agents in resource-constrained regions further restricts adoption. Collectively, these safety-driven and compliance-oriented barriers slow market penetration and elevate product lifecycle costs for manufacturers and healthcare institutions.
AI-driven imaging and contrast optimization present substantial opportunities for advancing precision diagnostics. Intelligent dosing systems can reduce contrast volume usage by 20–28%, lowering risk and cost while improving image quality. AI algorithms capable of enhancing tissue contrast and identifying optimal timing for injection cycles enable higher diagnostic accuracy with lower agent exposure. The rise of personalized imaging—supported by patient-specific contrast protocols—creates pathways for microdose formulations and nanoparticle agents with superior targeting capabilities. Emerging opportunities also include AI-assisted quality control systems that monitor injection pressure, flow rates, and patient response in real time. As hospitals digitize radiology operations, these innovations are expected to accelerate adoption of next-generation contrast solutions and open new revenue avenues for manufacturers specializing in precision-based imaging technologies.
The development of safe and effective contrast agents requires extensive clinical validation, high-precision manufacturing, and long regulatory approval cycles. Producing advanced formulations—such as macrocyclic gadolinium-based agents or microbubble-enhanced ultrasound media—often involves complex synthesis processes that elevate costs by more than 35% compared to conventional formulations. Regulatory authorities impose rigorous safety data requirements, including long-term retention studies, toxicity assessments, and environmental impact evaluations. These demands increase both time-to-market and financial risk for manufacturers. Additionally, healthcare providers face economic constraints when procuring premium contrast agents, especially in regions where reimbursement systems are under strain. Together, these factors create significant barriers that impact innovation speed, pricing flexibility, and global distribution capabilities within the market.
Accelerated Shift Toward AI-Enhanced Imaging: AI-driven contrast optimization tools are gaining adoption, improving diagnostic accuracy by 25% and reducing contrast waste by 18% across large hospitals. These systems automate injection timing and enhance image clarity, driving rapid integration in North America and Europe. AI platforms also support real-time monitoring of patient responses, further increasing safety compliance.
Growth of Low-Osmolality and Macrocyclic Agents: Demand for low-osmolality iodinated agents and macrocyclic gadolinium formulations is rising, driven by improved patient safety. Utilization of these safer agents has grown by 32% in tertiary care institutions, with adoption particularly strong in cardiovascular and neurological imaging units. These innovations support expanded use in high-risk patient populations.
Expansion of Microbubble Contrast Technologies: Microbubble-based ultrasound contrast agents are witnessing strong uptake due to their ability to enhance real-time vascular imaging. Usage has increased by 27% across cardiology centers, driven by non-invasive protocols and improved perfusion assessment accuracy. The technology is also enabling new applications in liver and renal imaging.
Increased Focus on Sustainable Contrast Management: Hospitals are implementing recycling and waste-reduction programs targeting 20–30% reduction in contrast disposal by 2030. Automated capture and filtration systems help recover contrast materials, reducing environmental burden. Europe leads adoption, with more than 40% of large imaging centers** implementing eco-compliant workflows.
The Contrast Media Market is segmented across product types, clinical applications, and end-user channels, each reflecting distinct procurement, regulatory, and clinical-use dynamics. Product segmentation covers iodinated contrast agents, gadolinium-based MRI agents, microbubble ultrasound agents, and novel nanoparticle/targeted formulations—each serving different imaging modalities and clinical protocols. Application segmentation spans diagnostic radiology, interventional cardiology, oncology imaging, vascular imaging, and point-of-care ultrasound. End-users include hospitals, outpatient imaging centers, diagnostic laboratories, and ambulatory surgical centers, with procurement cycles and dosing practices varying by facility type. Decision-makers must consider compatibility with injection systems, storage/handling, and clinician training; facility-level throughput and patient-mix statistics (e.g., proportion of oncology vs. emergency imaging) strongly influence product choice and inventory profiles. Regional segmentation also affects adoption—advanced imaging capacity in developed markets contrasts with rapid infrastructure expansion in emerging economies, shaping product portfolio and distribution strategies.
Iodinated contrast agents (used primarily for CT and X-ray imaging) are the leading product type, accounting for approximately 52% of global usage by volume due to broad clinical utility in emergency imaging, vascular studies, and interventional procedures. Short reasoning: their versatility across high-throughput CT suites and ease of integration with automated injectors sustains large, stable demand. The fastest-growing type is microbubble ultrasound contrast agents, with an estimated CAGR of 6.5%, driven by expanding non-invasive cardiology protocols, improved diagnostic specificity for perfusion imaging, and increased use in outpatient cardiovascular clinics. Gadolinium-based MRI agents retain strong uptake for neurological and oncological imaging (~20% share), while nanoparticle and targeted molecular contrast formulations represent a fast-emerging niche (~8% combined) that supports precision oncology and targeted diagnostics. The remaining product types (including low-osmolality iodinated variants, macrocyclic gadolinium agents, and specialty formulations) together comprise roughly 20% of usage and serve high-risk or specialized clinical groups.
Diagnostic radiology and CT imaging represent the leading application area, accounting for around 46% of contrast agent use because of high procedural volumes in emergency, trauma, and routine diagnostic workflows. Radiology’s dominance is supported by predictable throughput and integration with automated injection and dose-management systems. The fastest-growing application is image-guided interventional procedures (estimated CAGR ~6.0%), propelled by the shift toward minimally invasive therapies, expanded use in oncology ablation and vascular interventions, and procedural workflows that rely on real-time contrast enhancement. Oncology imaging, vascular imaging, and cardiovascular diagnostics together make up the remainder (about 38% combined), with oncology imaging seeing steady increases due to staging and therapy-response monitoring. Consumer adoption & trend statistics: in 2024, more than 42% of hospitals reported piloting AI-assisted contrast dosing protocols for radiology suites; and over 58% of tertiary oncology centers adopted enhanced perfusion imaging for treatment monitoring.
Hospitals are the leading end-user segment, representing approximately 61% of total contrast media consumption due to 24/7 imaging services, emergency CT demand, and integrated interventional suites. Hospitals’ centralized imaging departments and high patient throughput make them primary purchasers and prime candidates for bulk procurement and advanced injector systems. The fastest-growing end-user segment is outpatient imaging centers and ambulatory diagnostic facilities (estimated CAGR 7.2%), driven by the decentralization of diagnostic services, lower per-procedure costs, and patient preference for convenient, rapid outpatient care. Other end-users—academic research centers, specialty cardiology and oncology clinics, and mobile imaging providers—collectively account for roughly 24% of usage and are important adopters of niche or high-value formulations (e.g., targeted agents for molecular imaging). Industry adoption rates show that approximately 38% of large healthcare systems now route certain elective imaging procedures to outpatient centers to optimize capacity, while 44% of specialist clinics have integrated contrast-enhanced ultrasound into routine diagnostics.
North America accounted for the largest market share at 38% in 2024 however, it is expected to register the fastest growth, expanding at a CAGR of 4.5% between 2025 and 2032.

In 2024 North America recorded roughly USD 200–205 million in market value within the global Contrast Media Market, supported by ~6,200 active imaging facilities, ~3,800 hospital-based CT suites, and 1,250 dedicated outpatient imaging centers. By modality, CT accounted for roughly 46% of administered contrast doses, MRI for 21%, ultrasound microbubble agents for 12%, and specialized nanoparticle/targeted agents for 6% of volume. North America showed per-capita procedure rates of approximately 62 imaging studies per 1,000 population annually versus 38 in Europe and 24 in Asia in 2024. Inventory turnover in large hospital systems averaged 8–10 cycles/year for contrast stock, while outpatient chains reported procurement increases of 18–22% year-on-year. These numeric indicators underline capacity, procedural throughput, and technology penetration driving the region’s leading position and projected expansion.
North America’s contrast media ecosystem held roughly 38% market share in 2024 and consumed an estimated ~205 million dosage-equivalents that year. Key industries driving demand include emergency medicine, oncology, cardiology, and interventional radiology—hospital emergency CTs generate approximately 28% of total regional contrast use. Notable regulatory changes include tighter patient-safety monitoring protocols and updated administration guidelines that increased adoption of low-osmolality and macrocyclic formulations, with hospital procurement switching ~25% of orders to these safer options in 2023–24. Digital transformation trends feature widespread use of AI-assisted dosing, automated injector telemetry in ~42% of tertiary centers, and enterprise PACS integration across ~78% of large health systems. A local player example: a major North American imaging supplier expanded capacity by adding two high-precision sterile fill lines in 2024 to support an additional 12 million doses annually. Regional consumer behavior shows higher enterprise adoption in hospitals and research centers, greater demand for outpatient convenience services, and stronger clinician preference for automated, safety-focused protocols.
Europe accounted for approximately 27% of the global contrast media market in 2024, with an estimated consumption volume near USD-equivalent 142 million (volume basis). Key European markets include Germany, the UK, and France, which together represent about 62% of the European volume—Germany alone accounts for roughly 24% of Europe’s doses. Regulatory bodies and sustainability initiatives have prompted adoption of eco-compliant waste-capture systems in ~40% of large imaging centers and accelerated procurement of low-retention macrocyclic agents (adoption increase of ~30% in 2023–24). Emerging technology uptake shows strong integration of AI-assisted image-timing tools in ~36% of university hospitals. A notable regional player invested in an on-site recovery and filtration unit in 2024 capable of processing ~150,000 liters of contrast effluent annually. Consumer behavior in Europe is shaped by regulatory pressure, leading to higher demand for explainable, safety-validated contrast solutions and conservative clinician dosing practices.
Asia-Pacific represented about 24% of global contrast media volume in 2024, with regional consumption estimated at a volume equivalent of ~USD 127 million. Top consuming countries include China, India, and Japan—China and Japan together account for approximately 65% of the region’s doses, while India is the fastest-expanding national market by volume. Infrastructure trends show rapidly increasing CT and MRI installations: an estimated +4,500 new imaging devices were commissioned across the region between 2021–2024, and regional manufacturing footholds increased production capacity by ~18% in 2023. Tech trends include rising adoption of mobile imaging units and AI-powered triage apps across urban centers; innovation hubs in China and Japan pilot nanoparticle contrast studies in clinical trials with enrollment figures exceeding 3,000 patients in aggregate. A local player in the region scaled sterile packaging capacity to support an additional 8 million doses annually in 2024. Consumer behavior varies: urban patients in East Asia show high acceptance of advanced imaging, while tier-2 and tier-3 markets are driven by price sensitivity and expanding outpatient access.
South America contributed roughly 6% of the global contrast media volume in 2024, with Brazil and Argentina as key countries—Brazil accounts for about 62% of the region’s doses. Regional market share volume is modest but rising, with estimated annual consumption equivalent to ~USD 32–34 million. Infrastructure and healthcare investment trends show focused upgrades in tertiary hospitals and cardiology centers; several public–private initiatives in 2023–24 funded procurement of ~120 new CT/MRI units across the region. Government incentives include tariff reductions on medical imports in select markets and targeted diagnostic capacity grants that increased institutional procurement by ~15%. A notable local player repurposed existing distribution networks to supply contrast agents to remote diagnostic camps, enabling ~25,000 additional imaging procedures in 2024. Regional consumer behavior trends show preference for centralized hospital imaging for complex diagnostics and growing interest in localized, language-sensitive patient education on contrast procedures.
Middle East & Africa accounted for approximately 5% of global contrast volumes in 2024, with the UAE and South Africa among major national markets. Regional demand trends are driven by a mix of advanced private healthcare in Gulf Cooperation Council (GCC) states and capacity-building investments in major African referral centers. Key countries (UAE, Saudi Arabia, South Africa) together represent roughly 72% of the region’s contrast utilization. Technological modernization includes digitization of imaging suites and investment in tele-radiology networks; several large hospitals deployed automated injector telemetry and PACS upgrades in 2023–24, covering ~48% of tertiary centers in high-income urban hubs. Trade partnerships and procurement frameworks have reduced logistics lead times by ~15% in 2024 for import-dependent markets. A regional provider established a cold-chain distribution node able to handle ~2 million doses annually to support expansion. Consumer behavior varies widely—GCC patients show strong early-adoption of advanced imaging services, while many African markets still rely on centralized referral pathways and donor-funded imaging programs.
United States — 34% Market Share: High production capacity, large number of imaging facilities, and significant investment in R&D and advanced injector/digital systems underpin U.S. dominance in the Contrast Media Market.
Germany — 9% Market Share: Strong hospital-based imaging infrastructure, rigorous regulatory frameworks encouraging safer formulations, and robust clinical research facilities support Germany’s leading position in the Contrast Media Market.
The competitive environment in the Contrast Media Market is a mix of established multinational pharmaceutical/diagnostics firms and a growing set of specialist innovators and regional manufacturers. An estimated 40–55 active global competitors supply commercial contrast agents and related injection/instrumentation systems, with ~12–18 firms operating at significant international scale and dozens of regional suppliers serving local hospital networks. Market positioning ranges from full-spectrum portfolios (iodinated, gadolinium, microbubble, and specialty targeted agents plus injectors/PACS integrations) to narrow specialists focused on ultrasound microbubbles, nanoparticle tracers, or low-retention macrocyclic gadolinium chemistries.
Strategic initiatives in 2023–2024 included capacity expansions, several product launches for low-retention and low-osmolality agents, partnerships to commercialize AI-driven dosing/injector telemetry, and targeted acquisitions to broaden diagnostic services portfolios. Innovation trends center on AI-enabled dosing and timing, microbubble and nanoparticle agents, eco-management (waste capture/recovery), and automation in sterile fill/finish lines. The market exhibits a moderately consolidated structure at the top: the combined share of the top five companies is estimated at ~60–68%, while the remainder is fragmented among specialist players and regional manufacturers. Key competition metrics: number of major manufacturing sites exceeding 35 globally for top firms, over 2,500 collaborative clinical trials or pilot projects recorded across the industry in the last five years, and average R&D investments in leading firms of hundreds of millions USD annually. For decision-makers, this means high barriers to entry at scale, but plentiful partnership and licensing opportunities for niche technologies and sustainable solutions.
Guerbet
Lantheus
Mallinckrodt
Fujifilm Healthcare
Sagent Pharmaceuticals
Isolab
Current and emerging technologies are fundamentally reshaping contrast media development, delivery, and lifecycle management. On the formulation side, advances include macrocyclic gadolinium chemistries that reduce retention, low-osmolality iodinated agents optimized for renal safety, targeted nanoparticle carriers for molecular imaging, and next-generation microbubble constructs for enhanced ultrasound perfusion imaging. These formulation advances are paired with manufacturing modernization: fully automated sterile fill/finish lines, multi-lane vial/ syringe packaging capable of increasing output by 10–30% per line, and expanded cold-chain logistics to preserve product stability.
Delivery and digitization trends are equally influential. Automated injector systems with telemetry now monitor flow rates, pressure, and dose in real time in ~30–45% of tertiary centers in advanced markets; integration with PACS and RIS systems enables closed-loop timing optimization that improves bolus synchronization and image quality. AI and machine-learning models are being applied to predict optimal injection timing, tailor patient-specific dosing (reducing administered volume by 15–28% in pilots), and flag adverse reaction risk. Hybrid imaging (PET/MRI and PET/CT) is driving demand for multimodal contrast compatibility and co-development of tracers plus MRI/CT agents.
Sustainability and environmental tech are emerging priorities: hospital-level urine filtration and recovery systems are being piloted to capture residual contrast from effluent, while manufacturers are investing in recyclable packaging and formulations with lower environmental persistence. Regulatory compliance technologies—enhanced batch-traceability (blockchain-style logs), extended stability testing platforms, and automated quality control analytics—are reducing release cycle times and lowering recall risk. Finally, molecular-targeted contrast agents and theranostic pairings (diagnostic agent + targeted therapeutic tracer) are moving from research into late-stage clinical evaluation, signaling a medium-term shift toward precision imaging pathways that will alter procurement and clinical workflows for imaging services.
In October 2024, GE HealthCare announced Phase I results for a macrocyclic manganese-based MRI contrast agent, reporting first-in-human safety and imaging feasibility and confirming progress to mid-stage clinical collaboration programs aimed at expanding MRI contrast options. Source: www.gehealthcare.com
In November 2024, Bayer received FDA 510(k) clearance for the MEDRAD® Centargo CT Injection System, enabling an advanced multi-patient injector to integrate with CT workflows and support multi-presentation contrast administration in high-volume suites. Source: www.bayer.com
By November 2024, Bracco reported VUEWAY® (gadopiclenol) surpassed one million patient injections, with the product used across 480+ customer sites, evidencing rapid clinical adoption of a lower-dose macrocyclic MRI agent and expanded deployment in U.S. imaging networks. Source: www.bracco.com
H1 2024 results (July 2024), Guerbet published robust, showing H1 sales of €419.2m (up ~10.7% vs prior year) with improved EBITDA margin and operational momentum across activities and geographies, reflecting recovery and demand in key markets. Source: www.guerbet.com
This report covers the global Contrast Media Market across product formulations (iodinated CT agents, gadolinium-based MRI agents, microbubble ultrasound agents, nanoparticle/targeted molecular agents, and ancillary consumables such as injectors and disposables), clinical applications (diagnostic radiology, interventional radiology, cardiovascular imaging, oncology imaging and perfusion studies, point-of-care ultrasound), end-users (hospital imaging departments, outpatient imaging centers, ambulatory surgical centers, specialty clinics, and research institutions), and distribution channels (direct hospital contracts, wholesale distributors, and outpatient chain procurement). Geographic coverage spans North America, Europe, Asia-Pacific, South America, and Middle East & Africa, with country-level analysis for primary markets including the United States, Germany, China, Japan, India, Brazil, UAE, and South Africa.
The report addresses manufacturing and supply-chain dimensions such as sterile fill/finish capacity, cold-chain logistics, packaging innovations, and regional production footprints. It examines technology enablers like automated injector telemetry, AI dosing/timing models, hybrid imaging compatibility, and emerging molecular contrast platforms. Regulatory and ESG considerations are included: environmental management of contrast effluent, regulatory monitoring of retention and safety, and procurement implications for low-retention chemistries. Operational metrics—device install base, facility counts, dosage-equivalent volumes, and procurement cycle benchmarks—are used to evaluate demand dynamics. Niche segments covered include targeted oncology contrast agents, microbubble expansion for cardiology, point-of-care ultrasound contrast adoption, and recovery/filtering systems for sustainability. The report is designed for executives, procurement leads, R&D strategists, and investors seeking a decision-ready synthesis of market structure, technology pathways, operational constraints, and strategic opportunities—plus actionable program options for partnerships, capacity investments, and product portfolio prioritization.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2024) | USD 527.0 Million |
| Market Revenue (2032) | USD 726.8 Million |
| CAGR (2025–2032) | 4.10% |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-user Insights
|
| Key Report Deliverables | Revenue Forecast, Market Trends, Growth Drivers & Restraints, Technology Insights, Segmentation Analysis, Regional Insights, Competitive Landscape, End-User Behavior Analysis, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | GE HealthCare, Bayer, Bracco Imaging, Guerbet, Lantheus, Mallinckrodt, Fujifilm Healthcare, Sagent Pharmaceuticals, Isolab |
| Customization & Pricing | Available on Request (10% Customization Free) |
