Reports
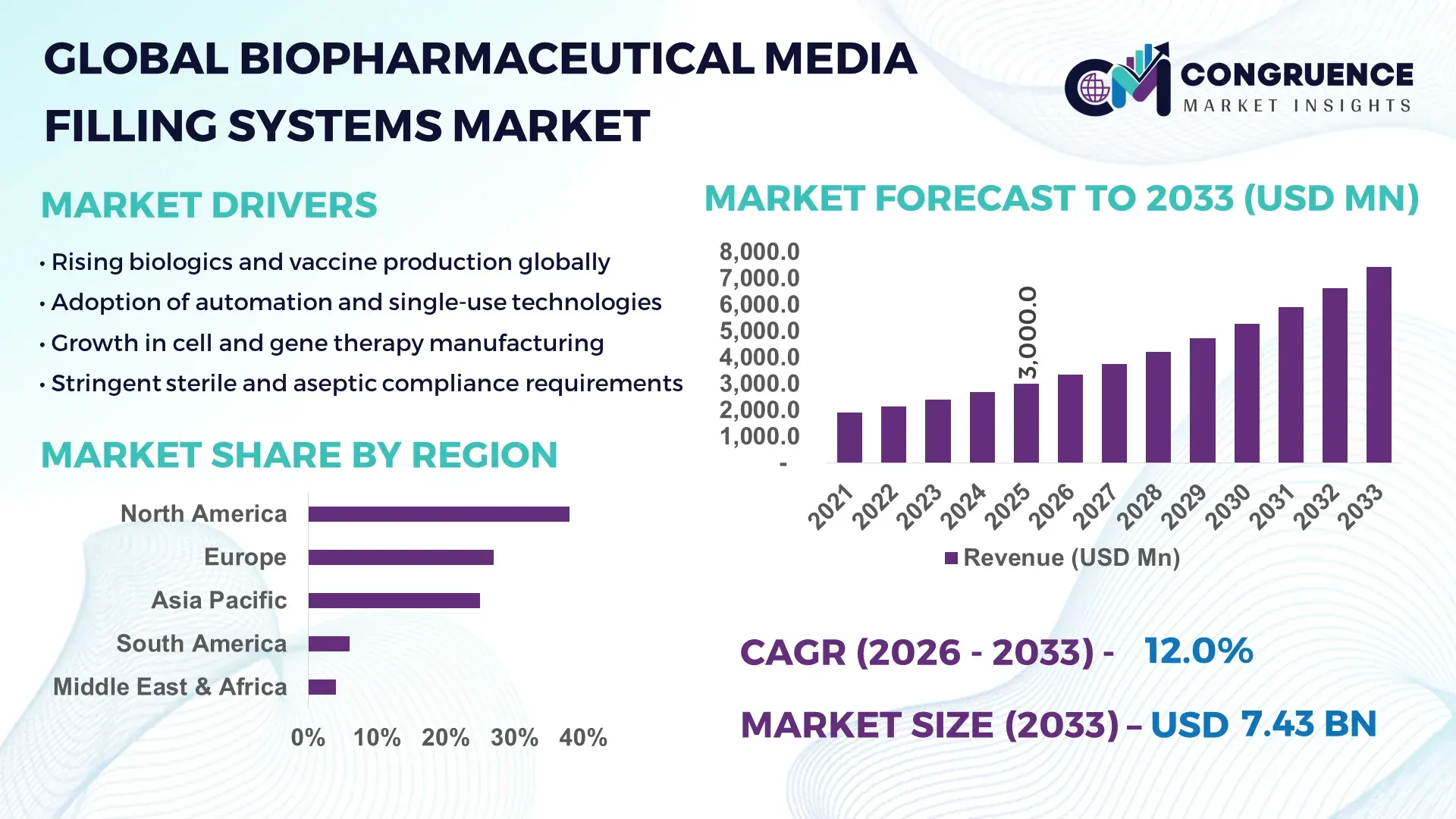
The Global Biopharmaceutical Media Filling Systems Market was valued at USD 3,000 Million in 2025 and is anticipated to reach a value of USD 7,427.9 Million by 2033 expanding at a CAGR of 12% between 2026 and 2033, according to an analysis by Congruence Market Insights. This growth is driven by the accelerating scale-up of biologics manufacturing and stricter sterility assurance requirements across global bioproduction facilities.
The United States hosts over 40% of global biomanufacturing capacity for sterile biologics, with more than 120 commercial-scale cell culture facilities actively using advanced media filling lines. Annual capital investment in bioprocess equipment exceeds USD 6–7 billion, with roughly 25% allocated to fill-finish and media preparation automation. U.S. plants increasingly deploy fully robotic isolator-based filling systems, processing 8,000–12,000 liters of culture media per batch. Leading applications include monoclonal antibodies, gene therapies, and mRNA vaccines, while digital aseptic monitoring and real-time contamination detection systems are now installed in nearly 65% of new facilities built since 2022.
Market Size & Growth: USD 3.0 billion in 2025 to USD 7.43 billion by 2033, with demand propelled by rising biologics production and zero-contamination manufacturing standards.
Top Growth Drivers: 68% increase in automated aseptic adoption; 32% reduction in contamination incidents; 27% improvement in line throughput from robotics.
Short-Term Forecast: By 2028, advanced isolator-based systems are expected to cut unplanned downtime by 22%.
Emerging Technologies: AI-driven sterility monitoring, single-use fluid pathways, and digital twin validation of filling lines.
Regional Leaders (2033): North America ~USD 2.8B (high automation intensity); Europe ~USD 2.1B (regulatory-led upgrades); Asia-Pacific ~USD 1.9B (capacity expansion surge).
Consumer/End-User Trends: Biopharma manufacturers prioritize closed systems and single-use media containers to minimize cleaning cycles.
Pilot/Case Example: In 2024, a U.S. facility integrated AI vision inspection, reducing manual QA intervention by 35%.
Competitive Landscape: Market leader holds ~28% share, followed by five global equipment suppliers with strong fill-finish portfolios.
Regulatory & ESG Impact: Stricter GMP rules and waste-minimization mandates are accelerating adoption of recyclable single-use components.
Investment & Funding Patterns: Over USD 4.5B invested globally since 2022 in automated aseptic infrastructure and modular plants.
Innovation & Future Outlook: Integration of predictive maintenance and real-time microbial analytics is redefining sterile operations.
Biopharmaceutical media filling systems serve biologics, vaccines, and cell-and-gene therapy sectors, with biologics accounting for roughly 55% of usage. Innovations such as single-use bags, robotic vial filling, and continuous monitoring have reduced batch failure risks. Regulations favor closed aseptic processing, while sustainability rules are pushing recyclable materials. North America leads in high-tech deployment, while Asia-Pacific grows fastest due to new manufacturing hubs and government incentives. Modular facilities and AI-driven quality control are set to reshape future capacity expansion.
Biopharmaceutical Media Filling Systems are becoming a strategic backbone of modern biomanufacturing as biologics replace traditional small-molecule drugs in therapeutic pipelines. These systems ensure sterility, consistency, and scalability—three pillars that determine whether a biologic can move from lab to commercial production. Governments and corporations are increasingly treating sterile fill-finish infrastructure as critical national health assets, particularly after pandemic-driven supply disruptions.
From a technology standpoint, AI-enabled aseptic monitoring delivers 28% fewer false contamination alerts compared to older manual particle-counting systems, allowing manufacturers to maintain speed without compromising quality. At the same time, single-use media pathways reduce cleaning validation time by nearly 40%, enabling faster batch turnover. This shift is transforming capital allocation: firms are investing more in intelligent automation rather than merely expanding floor space.
Regionally, North America dominates in production volume, supported by large-scale biologics plants, while Europe leads in adoption, with roughly 58% of enterprises using fully closed, single-use filling architectures driven by stringent regulatory expectations. Asia-Pacific, though behind in automation density, is rapidly scaling modular biomanufacturing parks that integrate media filling systems from day one.
In the short term, by 2028, AI-driven predictive maintenance is expected to improve overall equipment effectiveness (OEE) by 20%, reducing costly shutdowns and product losses. Compliance and ESG priorities are also reshaping strategies: major firms have pledged 30% reductions in plastic waste from single-use consumables by 2030 through recyclable polymers and material recovery programs.
A notable micro-scenario occurred in 2024, when a U.S.-based biologics manufacturer deployed autonomous robotic vial handlers across its media filling line, achieving 25% cycle-time reduction and 18% lower human intervention in critical cleanroom zones.
Looking ahead, the Biopharmaceutical Media Filling Systems Market will be central to resilient drug supply chains, ensuring sterile reliability, regulatory compliance, and sustainable growth in the era of precision medicine.
The Biopharmaceutical Media Filling Systems Market is shaped by rapid biologics expansion, stricter aseptic standards, and rising automation across sterile manufacturing. Manufacturers are shifting from conventional stainless-steel setups to flexible, single-use platforms that reduce cleaning complexity and contamination risk. Growing demand for vaccines, monoclonal antibodies, and cell therapies has intensified the need for high-throughput, closed filling lines. Meanwhile, digitalization—through real-time monitoring, AI inspection, and predictive maintenance—is redefining quality assurance practices. Supply chain localization, modular facility design, and government incentives for domestic bioproduction are further accelerating investments in modern media filling infrastructure worldwide.
Global biologics output has expanded sharply as monoclonal antibodies, recombinant proteins, and cell therapies move from niche treatments to mainstream medicine. Large-scale bioreactors exceeding 10,000 liters are now common, requiring equally advanced media preparation and sterile filling capabilities. Manufacturers are replacing manual processes with automated isolator-based systems to maintain consistency across high-volume batches. Cleanroom expansion in new bioparks has increased demand for precision filling lines that can handle diverse media formats, including single-use bags and flexible containers. Additionally, regulatory agencies are pushing for tighter aseptic control, prompting facilities to invest in closed-system media filling platforms that minimize human intervention and contamination risk.
Advanced media filling systems require significant upfront investment in isolators, robotics, cleanroom upgrades, and validation infrastructure. Many mid-sized biopharmaceutical firms struggle to justify these costs, particularly when product pipelines remain uncertain. Retrofitting legacy plants with modern aseptic technology can be more expensive than building new facilities, creating budget constraints. Moreover, specialized maintenance, skilled technicians, and software integration add ongoing operational costs. Smaller contract manufacturers often rely on refurbished equipment, which limits adoption of cutting-edge automation and slows overall market modernization despite clear technical benefits.
The rise of personalized and cell-based therapies is creating demand for smaller, more flexible media filling systems capable of handling multiple product types in the same facility. Modular, scalable lines allow manufacturers to switch rapidly between batches without extensive revalidation. Decentralized biomanufacturing hubs are emerging near hospitals and research centers, increasing the need for compact, high-precision filling equipment. Additionally, innovations in single-use technologies reduce cross-contamination risks and enable rapid production of patient-specific therapies, opening new revenue streams for equipment suppliers.
Evolving global GMP standards require continuous upgrades to media filling systems, increasing compliance burdens for manufacturers. Different regions impose varying validation requirements, complicating equipment standardization across multinational facilities. At the same time, there is a shortage of skilled aseptic engineers and cleanroom technicians, leading to delays in system installation and operation. Cybersecurity risks associated with digitalized filling lines add another layer of complexity, requiring robust protection measures that further increase operational costs and implementation timelines.
Modular cleanroom integration boosting deployment speed by 35%: Modular biomanufacturing facilities are increasingly pre-installed with media filling systems before site construction is complete, cutting project timelines by roughly 35%. Plug-and-play isolators reduce commissioning time, while prefabricated utility skids streamline installation. Over 55% of new biopharma projects now incorporate modular design elements, particularly in Europe and North America, where rapid capacity expansion is critical.
Robotics increasing throughput by 30% in sterile zones: Automated robotic vial handling and bag transfer systems are replacing manual operations in Grade A environments. Leading facilities report up to 30% higher throughput while reducing human presence in critical zones. Robotics also improves consistency in media dosing and container sealing, lowering variability across batches.
AI-based contamination monitoring lowering rejection rates by 25%: Real-time AI analytics integrated into filling lines can detect microbial or particulate anomalies within milliseconds. Early adopters have reduced batch rejection rates by approximately 25%, saving millions in wasted materials. Digital sterility dashboards are now standard in many high-end biomanufacturing plants.
Single-use media pathways cutting cleaning cycles by 40%: The shift from stainless steel to single-use fluid paths has reduced cleaning and sterilization cycles by up to 40%. This trend supports faster batch turnover, lower water usage, and reduced chemical waste. Adoption is strongest in North America, where sustainability and cost efficiency are top priorities for biopharma producers.
The Biopharmaceutical Media Filling Systems Market is structured around equipment types, applications, and end-users that reflect the operational realities of modern biomanufacturing. By type, the market spans automated, semi-automated, and manual systems, as well as specialized isolator-based and robotic platforms that differ in precision, throughput, and sterility assurance. Application-wise, demand is concentrated in biologics, vaccines, and cell-and-gene therapies, with varying process requirements for liquid media, buffer preparation, and sterile filling. End-user segmentation centers on biopharmaceutical manufacturers, contract development and manufacturing organizations (CDMOs), academic and research institutions, and government laboratories. Across all segments, adoption is increasingly shaped by cleanroom classification, batch scale, contamination risk tolerance, and the need for operational flexibility in multiproduct facilities.
Automated media filling systems represent the largest portion of the market, accounting for roughly 48% of current installations, driven by high-throughput performance, minimal human intervention, and consistent sterility control in Grade A/B environments. These systems integrate robotic vial handling, closed transfer lines, and real-time particle monitoring, making them the default choice for large commercial biologics plants that run continuous or semi-continuous operations.
Semi-automated systems currently hold about 27% adoption, favored by mid-sized facilities that balance automation with lower capital intensity and greater operator flexibility. Manual or conventional filling systems, along with niche hybrid platforms, together represent the remaining 25% of the market and are primarily used in pilot plants, early-stage R&D sites, or legacy facilities where full automation is not yet justified.
The fastest-expanding category is isolator-based robotic filling systems, growing at roughly 14% annually, fueled by stricter aseptic regulations, rising biologics batch complexity, and the need to minimize cleanroom personnel. These systems reduce human presence in critical zones, shorten validation cycles, and enable remote monitoring, which is increasingly valuable in high-containment bioprocessing.
Other specialized types—such as single-use fluid path systems and modular skid-mounted fillers—play a smaller but strategically important role by supporting rapid facility deployment and multiproduct flexibility.
In 2025, a major European biologics producer deployed fully robotic isolator filling lines that processed over 9,000 liters of culture media per batch while cutting manual interventions by more than 60%.
Biologics manufacturing is the leading application, representing about 46% of market usage, because monoclonal antibodies and recombinant proteins require stringent sterility controls and high-volume media handling. Vaccine production follows closely at roughly 29%, supported by surge capacity requirements and government-backed facility upgrades after recent pandemic preparedness initiatives.
The fastest-rising application is cell and gene therapy media filling, expanding at around 15% per year, as personalized therapies demand smaller batch sizes, rapid turnaround, and highly flexible filling configurations that can switch between products with minimal revalidation.
Other applications—such as buffer preparation, diagnostic reagent filling, and sterile water for injection—collectively account for the remaining 25%, serving as essential but lower-volume use cases within multiproduct plants.
Adoption patterns show that in 2025, more than 40% of large biopharma enterprises were piloting next-generation closed media filling platforms to support multiproduct pipelines. Separately, over 58% of contract manufacturers reported prioritizing single-use media systems to reduce cleaning downtime and cross-contamination risk.
In 2025, a global vaccine manufacturer implemented AI-assisted sterile media filling across three sites, enabling real-time contamination detection and reducing batch rejections by approximately 30% during seasonal production surges.
Biopharmaceutical manufacturers are the dominant end-users, accounting for roughly 52% of system deployments, as large integrated producers require high-capacity, fully automated media filling lines to support continuous biologics production. These firms typically operate multiple commercial plants and invest heavily in digital quality systems, robotics, and predictive maintenance.
The fastest-growing end-user segment is CDMOs, expanding at about 13% annually, driven by outsourcing trends, rising demand for contract biologics production, and the need for flexible multiproduct filling capabilities. CDMOs increasingly prefer modular, single-use media systems that allow rapid client onboarding and faster facility turnover.
Academic and research institutions, together with government laboratories, make up the remaining 23% of the market. While lower in volume, these users are important innovation hubs that test new aseptic technologies and influence future industry standards. Adoption rates are particularly high in translational research centers, where roughly 45% of leading labs now use semi-automated or hybrid media filling platforms to bridge discovery and pilot-scale manufacturing.
In 2025, about 37% of global biomanufacturing enterprises reported piloting advanced closed media filling systems to improve sterility assurance. Meanwhile, nearly 55% of North American research institutes indicated plans to upgrade to isolator-based platforms within two years.
In 2025, a national biomedical research center integrated robotic media filling into its cell-therapy pilot facility, cutting cleanroom staffing requirements by 35% while maintaining full GMP compliance.
North America accounted for the largest market share at 38% in 2025; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 14% between 2026 and 2033.

The global distribution of the Biopharmaceutical Media Filling Systems Market reflects both maturity and expansion dynamics. North America leads due to its established biologics manufacturing base, followed by Europe at 27%, supported by strong regulatory-driven upgrades. Asia-Pacific represents 25% of the market, driven by rapid capacity expansion in China, India, and Japan. South America accounts for 6%, while the Middle East & Africa holds 4%, reflecting emerging infrastructure development. Increasing investments in sterile manufacturing, government-backed biopharma programs, and automation adoption rates above 60% in developed regions continue to reshape regional demand patterns, while emerging regions focus on scaling local production and reducing import dependency.
North America holds approximately 38% of the global market, driven by high biologics output and advanced manufacturing infrastructure. Key demand originates from monoclonal antibodies, vaccines, and gene therapy production. Regulatory frameworks emphasize strict aseptic compliance, encouraging adoption of isolator-based and robotic filling systems. Government-backed funding programs support domestic biomanufacturing resilience and capacity expansion. Digital transformation is evident, with over 65% of new facilities integrating AI-based contamination monitoring. Local equipment suppliers are focusing on closed-system, single-use compatible platforms to reduce downtime. Regional adoption behavior shows higher enterprise uptake in healthcare and life sciences, with automation penetration exceeding 70% in large-scale plants.
Europe represents 27% of the global market, with Germany, the UK, and France as leading contributors. Stringent regulatory oversight and sustainability mandates drive upgrades toward energy-efficient, low-waste filling systems. Regulatory bodies promote single-use technologies to reduce water and chemical consumption. Adoption of digital batch monitoring and electronic batch records exceeds 55% across Western Europe. Regional players are investing in modular filling skids to support multiproduct operations. Consumer behavior reflects strong regulatory influence, with manufacturers prioritizing explainable, validated automation to meet compliance and audit requirements.
Asia-Pacific accounts for 25% of global demand, ranking third by share but first by growth momentum. China, India, and Japan lead consumption due to aggressive biologics capacity build-out. Large-scale bioparks and contract manufacturing hubs are expanding sterile filling lines at double-digit installation rates. Regional innovation hubs emphasize cost-efficient automation and localized equipment production. Local players are scaling modular and prefabricated filling units to accelerate plant commissioning. Adoption behavior highlights strong uptake among emerging biopharma firms, with over 50% prioritizing automation to meet export-quality standards.
South America holds 6% of the global market, led by Brazil and Argentina. Government initiatives encourage domestic vaccine and biologics manufacturing to reduce imports. Infrastructure modernization in pharmaceutical plants is driving steady adoption of semi-automated filling systems. Trade incentives support technology transfer and equipment upgrades. Regional players focus on flexible, mid-scale systems suited for public health production. Consumer behavior indicates demand tied to localized biologics and regional language labeling requirements.
The Middle East & Africa region accounts for 4% of global demand, with growth concentrated in the UAE and South Africa. Governments are investing in healthcare diversification and local drug manufacturing. Technological modernization includes adoption of closed filling systems to meet international quality standards. Trade partnerships facilitate equipment imports and joint ventures. Regional players emphasize turnkey filling solutions for new facilities. Adoption behavior shows growing interest in automation to reduce reliance on imports and enhance supply security.
United States – 31% Market Share: High biologics production capacity and extensive investment in automated sterile manufacturing facilities.
Germany – 11% Market Share: Strong regulatory-driven modernization and advanced pharmaceutical engineering capabilities supporting aseptic processing.
The Biopharmaceutical Media Filling Systems Market exhibits a moderately consolidated competitive environment with a mix of global heavyweights and specialized equipment manufacturers addressing diverse biopharmaceutical aseptic filling needs. There are over 60 active competitors worldwide offering solutions ranging from automated isolator-based fillers to modular and single-use media handling platforms. Leading players have collectively captured approximately 38–42% of total market share, indicating significant presence but still leaving room for emerging innovators. The competitive landscape is shaped by strategic initiatives such as product launches, partnerships, acquisitions, and technological innovation. For example, Stevanato Group expanded its sterile processing capabilities through acquisition of Sepha Ltd in March 2025, while Bosch Packaging Technology formed a partnership with Coesia in May 2025 to co-develop digitally integrated aseptic fill lines. Innovation trends include integration of AI-based quality monitoring, real-time particle detection, and robotics to enhance throughput and contamination control. Regional competition is strongest in North America and Europe, where firms leverage advanced R&D facilities and robust supply chains to introduce next-generation aseptic media filling systems with improved precision and flexibility. As biopharmaceutical pipelines evolve toward complex biologics, CDMOs and equipment manufacturers are intensifying collaborations to ensure tailored sterile solutions across scales and modalities, expanding both portfolio depth and geographical reach.
Bosch Packaging Technology
Optima Packaging Group GmbH
Groninger & Co. GmbH
Marchesini Group S.p.A.
Vanrx Pharmasystems Inc.
SP Scientific Products (SP Industries, Inc.)
Romaco Group
Cozzoli Machine Company
Tofflon Science and Technology Co., Ltd.
Watson-Marlow Fluid Technology Group
Aseptic Technologies
Colanar Inc.
The Biopharmaceutical Media Filling Systems Market is undergoing a technological transformation driven by the need for higher sterility assurance, reduced contamination risk, and improved operational efficiency. Automation and robotics are at the forefront, enhancing throughput in aseptic environments by minimizing manual intervention in critical areas. Latest systems incorporate robotic vial handling and automated media transfer units capable of maintaining closed sterile conditions throughout the process. Digital technologies such as AI-powered inline quality monitoring and machine vision are increasingly embedded into filling platforms to detect particulates or anomalies in real time, reducing defect rates and improving batch reliability. Modular and single-use technologies are gaining traction, particularly for flexible production lines that support small-batch and multiproduct biopharmaceutical manufacturing without extensive cleaning or validation cycles. These single-use fluid pathways also reduce turnaround time between batches and help meet strict regulatory cleanliness requirements. Isolator–based containment systems are becoming standard in high-potency biologics and advanced therapy applications, offering a barrier that significantly lowers operator exposure and contamination risk. Integration with real-time data analytics and digital twins allows predictive maintenance and optimization of process parameters, extending equipment uptime and reducing unscheduled downtime. Stakeholders are investing in end-to-end digital ecosystems that link filling systems with upstream media preparation and downstream packaging for seamless data integrity and compliance. As demand for complex biologics, vaccines, and cell therapies rises, these advanced technologies provide the flexibility and precision necessary to support diverse production scenarios, from clinical batch runs to full commercial supply, ensuring media filling systems remain robust, compliant, and future-ready in evolving manufacturing landscapes.
• In May 2025, Syntegon unveiled its SynTiso line concept for liquid pharmaceutical filling at Pharmatag 2025 in Crailsheim, Germany, featuring a fully automated, gloveless isolator with contactless suspended transport capable of processing up to 600 syringes, vials, or cartridges per minute, enhancing sterility and reducing contamination risk. Source: www.syntegon.com
• In September 2025, Syntegon Technology GmbH reported strong H1 2025 performance, with sales of €824 million (+11%) and EBITDA up 41% to €127 million, driven by robust demand for aseptic, isolator-equipped fill & finish solutions and continued strategic execution. Source: www.syntegon.com
• In April 2025, Steriline showcased its advanced robotic vial filling machine using Planar Motor technology at INTERPHEX 2025 in New York, offering six-degree precise motion and zero friction transport for improved contamination control and packaging flexibility. Source: www.worldpharmatoday.com
• In March–April 2025, multiple key market players, including Syntegon and Steriline, participated in Pharmatag and industry expos, highlighting innovations in aseptic processing, collaborative development of bespoke filling solutions, and strategic partnerships to address evolving pharmaceutical manufacturing needs. Source: www.syntegon.com
The Biopharmaceutical Media Filling Systems Market Report provides comprehensive coverage of market segments, technologies, end uses, and geographic landscapes that define current and future trends in sterile bioprocessing. It encompasses detailed analysis of equipment types, including automated, semi-automated, isolator-based, and single-use systems, addressing diverse biopharmaceutical needs from clinical development to large-scale commercial production. Geographic insights span key regions such as North America, Europe, Asia-Pacific, South America, and Middle East & Africa, offering segmentation of regional demand dynamics and investment trends. The report details end-user categories including biopharmaceutical manufacturers, CDMOs (contract development and manufacturing organizations), research institutions, and government labs, highlighting adoption patterns in biologics, vaccines, and advanced therapies. Technology segments explore robotics, AI-enabled inline monitoring, predictive maintenance, and modular cleanroom integration, illustrating innovation footprints influencing competitive positioning. Additionally, the report addresses industry focus areas such as regulatory compliance environments, sustainability drivers like single-use waste reduction strategies, and digitalization for quality and data integrity. Niche segments such as small-batch personalized medicine filling, advanced therapy media handling, and flexible production lines are also examined, reflecting evolving needs toward personalized and precision biologics. Structured for strategic decision-makers, the report synthesizes statistical data, market share metrics, technology trends, and competitive moves to support investment planning, procurement decisions, and product strategy formulation tailored to this critical segment of biopharmaceutical manufacturing.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2025) | USD 3,000.0 Million |
| Market Revenue (2033) | USD 7,427.9 Million |
| CAGR (2026–2033) | 12.0% |
| Base Year | 2025 |
| Forecast Period | 2026–2033 |
| Historic Period | 2021–2025 |
| Segments Covered |
By Type
By Application
By End-User Insights
|
| Key Report Deliverables | Revenue Forecast, Market Trends, Growth Drivers & Restraints, Technology Insights, Segmentation Analysis, Regional Insights, Competitive Landscape, Regulatory & ESG Overview, Recent Developments |
| Regions Covered | North America; Europe; Asia-Pacific; South America; Middle East & Africa |
| Key Players Analyzed | Syntegon Technology GmbH, IMA Group, Bausch + Ströbel, Bosch Packaging Technology, Optima Packaging Group GmbH, Groninger & Co. GmbH, Marchesini Group S.p.A., Vanrx Pharmasystems Inc., SP Scientific Products (SP Industries, Inc.), Romaco Group, Cozzoli Machine Company, Tofflon Science and Technology Co., Ltd., Watson-Marlow Fluid Technology Group, Aseptic Technologies, Colanar Inc. |
| Customization & Pricing | Available on Request (10% Customization Free) |
