Reports
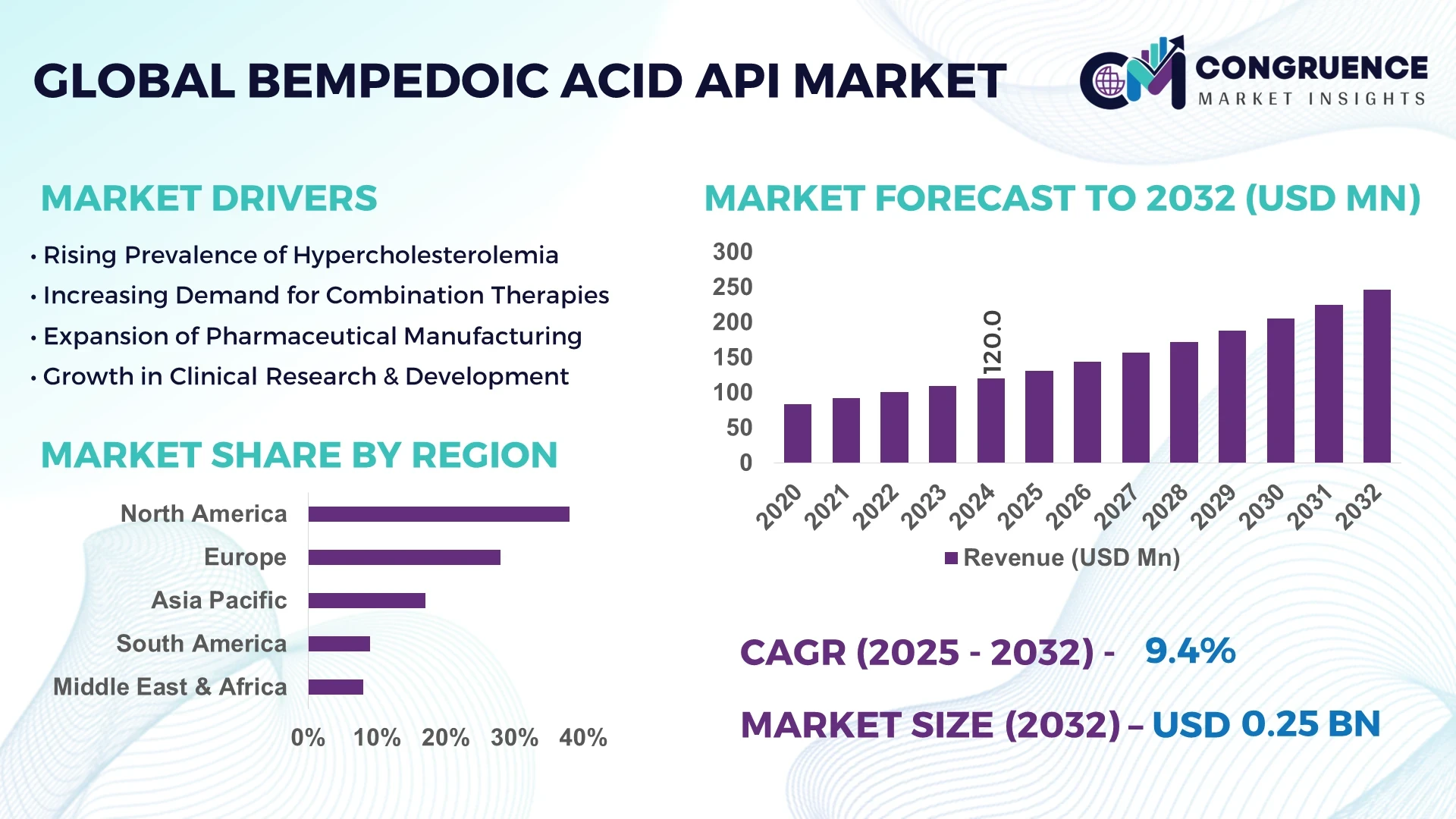
The Global Bempedoic Acid API Market was valued at USD 120.0 Million in 2024 and is anticipated to reach a value of USD 246.2 Million by 2032, expanding at a CAGR of 9.4% between 2025 and 2032.
The United States stands as a pivotal player in the Bempedoic Acid API market, characterized by substantial production capacities, significant investments in pharmaceutical manufacturing, and a robust pipeline of lipid-lowering therapies.
The Bempedoic Acid API market is experiencing notable growth, driven by the increasing prevalence of hypercholesterolemia and the demand for non-statin cholesterol-lowering therapies. Advancements in drug formulation technologies and the development of fixed-dose combination therapies are enhancing the efficacy and patient compliance of treatments. North America leads the market, with a significant share attributed to established healthcare infrastructure and high adoption rates of innovative therapies. Emerging markets, particularly in Asia-Pacific, are witnessing rapid growth due to rising healthcare investments and the escalating burden of cardiovascular diseases. Regulatory support and the introduction of generic formulations are further propelling market expansion, making Bempedoic Acid a cornerstone in managing lipid-related disorders.
Artificial Intelligence (AI) is revolutionizing the Bempedoic Acid API market by streamlining drug discovery processes, optimizing manufacturing efficiencies, and enhancing personalized treatment approaches. AI-driven algorithms are expediting the identification of potential drug candidates, reducing the time and cost associated with research and development. In manufacturing, AI technologies are being employed to monitor production lines in real-time, ensuring consistent quality and minimizing waste. Additionally, AI facilitates the analysis of patient data to tailor personalized treatment regimens, improving therapeutic outcomes. These advancements not only accelerate the development and delivery of Bempedoic Acid-based therapies but also contribute to cost reductions and enhanced patient satisfaction.
"In 2024, a leading pharmaceutical company implemented an AI-based predictive maintenance system in its Bempedoic Acid API production facility, resulting in a 30% reduction in equipment downtime and a 15% increase in overall production efficiency."
The Bempedoic Acid API market is influenced by various dynamic factors, including technological advancements, regulatory developments, and shifting healthcare needs. The demand for non-statin therapies is growing, driven by patient preferences and the need for alternative treatment options. Innovation in drug formulations and delivery methods is enhancing the therapeutic efficacy of Bempedoic Acid, while regulatory approvals are facilitating market access. Economic factors, such as healthcare expenditure and insurance coverage, also play a crucial role in shaping market dynamics.
The rising incidence of hypercholesterolemia globally is a significant driver for the Bempedoic Acid API market. With an estimated 39.8% of adults affected by high cholesterol levels, the demand for effective cholesterol-lowering therapies is escalating. Bempedoic Acid, as a non-statin alternative, offers a promising solution for patients intolerant to traditional statin therapies, thereby expanding its market potential.
Stringent regulatory requirements and lengthy approval processes pose challenges to the timely introduction of new Bempedoic Acid formulations. Delays in obtaining regulatory approvals can hinder market entry and affect the availability of innovative therapies, impacting market growth.
Emerging markets, particularly in Asia-Pacific and Latin America, present significant growth opportunities for the Bempedoic Acid API market. Increasing healthcare investments, rising awareness of cardiovascular diseases, and expanding access to healthcare services are driving the demand for lipid-lowering therapies in these regions. Strategic partnerships and localized production can facilitate market penetration and capitalize on this growth potential.
The high cost of Bempedoic Acid therapies remains a challenge, particularly in low- and middle-income countries. Ensuring cost-effectiveness and affordability is crucial to expanding access to these treatments. Manufacturers are exploring strategies such as generic formulations and cost-reduction technologies to address this challenge and make therapies more accessible to a broader patient population.
Rise in Modular and Prefabricated Construction: The adoption of modular construction is reshaping demand dynamics in the Bempedoic Acid API Market. Pre-bent and cut elements are prefabricated off-site using automated machines, reducing labor needs and speeding project timelines. Demand for high-precision machines is rising, especially in Europe and North America, where construction efficiency is critical.
Advancements in Drug Formulation Technologies: Innovations in drug delivery systems, such as sustained-release formulations and combination therapies, are enhancing the efficacy and patient compliance of Bempedoic Acid treatments. These advancements are expanding the therapeutic applications and market reach of Bempedoic Acid API.
Regulatory Support for Non-Statin Therapies: Regulatory agencies are providing expedited approval pathways for non-statin lipid-lowering therapies, including Bempedoic Acid. This support is facilitating faster market access and encouraging investment in the development of alternative cholesterol-lowering treatments.
Integration of AI in Drug Discovery and Manufacturing: The integration of Artificial Intelligence in drug discovery and manufacturing processes is streamlining operations and accelerating the development of Bempedoic Acid therapies. AI-driven approaches are improving efficiency, reducing costs, and enhancing the precision of drug formulations.
The Bempedoic Acid API Market is strategically segmented into types, applications, and end-users, providing a comprehensive understanding of market dynamics. Type segmentation identifies variations in chemical composition, purity levels, and formulation methods, each catering to distinct therapeutic and production requirements. Applications are differentiated based on the final therapeutic use of Bempedoic Acid, such as standalone cholesterol-lowering therapy or inclusion in combination medications. End-user segmentation focuses on the entities utilizing the API, including pharmaceutical manufacturers, contract research organizations, and biotech firms. This structured segmentation allows industry decision-makers to identify priority areas for investment, innovation, and strategic expansion, while also highlighting growth opportunities in emerging markets, technology integration, and evolving regulatory environments. Insights into each segment reveal consumption patterns, production priorities, and supply-chain considerations critical for competitive positioning.
The Bempedoic Acid API Market includes several key product types, with the standard pharmaceutical-grade Bempedoic Acid API being the leading type due to its widespread adoption in cardiovascular drug formulations. This type is extensively used by manufacturers producing oral lipid-lowering therapies and fixed-dose combinations, offering a high level of purity and stability suitable for large-scale production. The fastest-growing type is the modified-release API variant, driven by increasing demand for formulations that enhance patient compliance and enable controlled drug delivery. Other types, such as high-purity experimental-grade APIs and combination-ready intermediates, serve niche applications including research laboratories, early-phase clinical trials, and specialized therapeutic formulations. Each type contributes uniquely to the overall market, with variations in synthesis methods, particle size distribution, and formulation compatibility catering to diverse industry requirements.
Application-wise, the Bempedoic Acid API Market is primarily focused on cardiovascular therapies, with standalone cholesterol-lowering drugs representing the leading application due to the large population of patients requiring non-statin treatments. Fixed-dose combination therapies, combining Bempedoic Acid with other lipid-lowering agents, represent the fastest-growing application segment, supported by trends in personalized medicine and enhanced patient adherence. Other applications include incorporation into pharmaceutical intermediates for specialty cardiovascular medications and investigational combination therapies in clinical trials. Pharmaceutical manufacturers leverage these applications to optimize therapeutic outcomes and differentiate products, while contract research organizations utilize APIs for experimental formulations. Market growth is reinforced by rising demand for non-statin alternatives, expanding healthcare infrastructure, and technological advances in drug delivery systems.
The leading end-user segment in the Bempedoic Acid API Market comprises pharmaceutical manufacturers producing commercial lipid-lowering medications. These manufacturers utilize bulk API for formulation into tablets, capsules, or combination therapies. The fastest-growing end-user segment is contract research organizations (CROs) and biotech firms, which are increasingly integrating Bempedoic Acid API into clinical trial pipelines, early-stage research, and novel drug development projects. Hospitals, academic institutions, and specialty laboratories also represent smaller but important end-users, contributing to the market through experimental and investigational applications. The segmentation by end-user provides insight into demand distribution, procurement trends, and strategic collaboration opportunities, offering decision-makers a clear understanding of where production, supply, and innovation efforts should be prioritized.
North America accounted for the largest market share at 38% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 9.4% between 2025 and 2032.
North America continues to lead due to advanced pharmaceutical manufacturing infrastructure, early adoption of innovative lipid-lowering therapies, and a robust healthcare network supporting research and production of Bempedoic Acid API. Meanwhile, Asia-Pacific is rapidly developing, driven by expanding healthcare access, rising prevalence of hypercholesterolemia, and increasing investments in API manufacturing facilities. Europe, South America, and the Middle East & Africa contribute smaller portions but are witnessing steady demand growth due to emerging healthcare initiatives and technological adoption. Globally, production capacity, regulatory support, and technological advancements are shaping the regional distribution and market expansion opportunities in the Bempedoic Acid API sector.
North America held approximately 38% of the global Bempedoic Acid API market in 2024. The demand is primarily driven by the pharmaceutical and biotech industries, focusing on cardiovascular disease treatments. Regulatory frameworks, including FDA approvals, facilitate faster commercialization of new formulations, while government initiatives encourage domestic API production. Technological advancements, such as automated synthesis and AI-assisted process optimization, are enhancing manufacturing efficiency and quality control. Additionally, digital platforms and predictive analytics are increasingly integrated into production and supply-chain management, enabling manufacturers to respond swiftly to market demand. The region also benefits from a well-established distribution network ensuring timely delivery of Bempedoic Acid API to key therapeutic markets.
Europe accounted for nearly 28% of the global Bempedoic Acid API market in 2024, with Germany, the UK, and France being the leading contributors. European pharmaceutical manufacturers emphasize compliance with stringent EMA regulations and sustainability initiatives promoting green chemistry in API production. The adoption of advanced technologies, including continuous-flow synthesis and process analytical technology (PAT), has improved efficiency and product quality. Investments in R&D centers and collaborations with academic institutions accelerate development of novel formulations. Rising demand in cardiovascular healthcare, combined with regulatory support and regional healthcare infrastructure, positions Europe as a critical hub for Bempedoic Acid API production and innovation.
Asia-Pacific ranked third in market volume in 2024, with China, India, and Japan as the top consuming countries. Expanding pharmaceutical manufacturing infrastructure and government incentives for local API production are key drivers. The region is witnessing adoption of advanced process technologies, including high-purity synthesis, automation, and AI-assisted process monitoring. Emerging innovation hubs in India and China are contributing to faster development of cost-effective Bempedoic Acid API. Rising prevalence of cardiovascular disorders, coupled with improving healthcare access, is fueling regional consumption. Partnerships between multinational companies and local manufacturers are enhancing technology transfer and market penetration.
South America contributed approximately 12% to the global Bempedoic Acid API market in 2024, with Brazil and Argentina as key markets. The region benefits from improving pharmaceutical infrastructure and growing demand for cholesterol-lowering therapies. Government incentives and trade policies are supporting the import of advanced APIs and local production initiatives. Expansion of healthcare networks and increasing awareness of cardiovascular diseases are boosting demand. Manufacturers are investing in advanced production technologies and quality-control measures to meet regulatory compliance and supply regional therapeutic markets effectively.
Middle East & Africa accounted for around 10% of the market in 2024, with the UAE and South Africa leading growth. Demand is driven by pharmaceutical expansion, healthcare modernization, and increasing prevalence of lifestyle-related cardiovascular conditions. Technological modernization trends, including automated API synthesis and digital supply-chain management, are being adopted by regional manufacturers. Regulatory support, trade partnerships, and investment in healthcare infrastructure facilitate access to advanced Bempedoic Acid therapies. The region shows growing potential as local production capabilities improve and multinational companies expand operations.
United States – 38% Market Share
High production capacity and early adoption of innovative lipid-lowering therapies.
Germany – 12% Market Share
Strong pharmaceutical manufacturing infrastructure and regulatory support for advanced API production.
The Bempedoic Acid API Market is highly competitive, featuring over 25 active global players that focus on innovation, production capacity expansion, and strategic partnerships to maintain market positioning. Leading competitors differentiate themselves through investments in high-purity API manufacturing, advanced synthesis technologies, and enhanced quality control measures. Strategic initiatives include collaborative agreements between pharmaceutical companies and contract manufacturers to optimize production efficiency and reduce supply chain risks. Product launches, including modified-release APIs and combination-ready intermediates, are reshaping competition by addressing patient compliance and therapeutic needs. Additionally, mergers and acquisitions among mid-sized API manufacturers are consolidating expertise and technological capabilities, enhancing market reach. Innovation trends, such as AI-driven process optimization, continuous-flow synthesis, and automation in quality monitoring, are crucial factors influencing competitive dynamics. Companies are also leveraging digital platforms for regulatory tracking, real-time production analytics, and predictive maintenance, ensuring operational resilience. Overall, the market environment is shaped by technological leadership, capacity expansion, and strategic alliances, requiring active competitors to continuously innovate and optimize production processes.
Esperion Therapeutics, Inc.
Lonza Group AG
Apotex Inc.
Hovione LLC
Zhejiang Hisun Pharmaceutical Co., Ltd.
Teva Pharmaceutical Industries Ltd.
WuXi AppTec Co., Ltd.
Samsung Biologics Co., Ltd.
Dr. Reddy’s Laboratories Ltd.
Ajanta Pharma Ltd.
The Bempedoic Acid API Market is witnessing significant technological advancements that are shaping production, quality control, and R&D processes. Continuous-flow synthesis technology is increasingly employed to enhance reaction efficiency, reduce impurities, and ensure batch-to-batch consistency. Automation in manufacturing lines and AI-driven predictive maintenance systems improve operational uptime, optimize process parameters, and lower human error risks. Advanced purification techniques, including crystallization and chromatography, are being integrated to achieve higher purity APIs suitable for pharmaceutical formulations. Analytical technologies, such as high-performance liquid chromatography (HPLC) and near-infrared spectroscopy, are utilized for real-time monitoring of API quality. Additionally, the adoption of digital twins and process simulation allows manufacturers to forecast performance, troubleshoot issues virtually, and enhance production scalability. Emerging technologies, such as microreactor platforms, facilitate faster and safer synthesis of complex molecules while minimizing chemical waste. Companies are also exploring eco-friendly solvents and green chemistry approaches to align with environmental standards. These technological innovations collectively improve manufacturing efficiency, product quality, and regulatory compliance, supporting the expansion of Bempedoic Acid API production across global markets.
In March 2023, Lonza Group expanded its API manufacturing facility in Switzerland, incorporating continuous-flow synthesis equipment that increased production efficiency by 20% while reducing operational downtime.
In September 2023, Esperion Therapeutics introduced a modified-release Bempedoic Acid API designed for enhanced patient adherence in combination therapies, achieving greater stability in high-temperature storage conditions.
In January 2024, WuXi AppTec implemented an AI-assisted predictive maintenance system in its API production units, leading to a 15% reduction in unscheduled equipment downtime and optimizing resource utilization.
In June 2024, Hovione LLC upgraded its purification processes using advanced crystallization techniques, resulting in a 12% improvement in API purity and reduced impurity levels for commercial-scale production.
The Bempedoic Acid API Market Report provides a comprehensive analysis of the global API landscape, covering detailed insights across product types, applications, and end-users. The report examines standard pharmaceutical-grade APIs, modified-release variants, and combination-ready intermediates, highlighting their industrial relevance and adoption in cardiovascular therapies. It evaluates key therapeutic applications, including standalone cholesterol-lowering medications and inclusion in combination therapies, providing decision-makers with actionable intelligence for portfolio development. The geographic scope includes North America, Europe, Asia-Pacific, South America, and the Middle East & Africa, analyzing market volume, growth ranking, and regional production and consumption trends. The report emphasizes technological innovations such as continuous-flow synthesis, automation, AI-driven process optimization, and advanced purification methods that impact efficiency, quality, and scalability. Industry focus areas include pharmaceutical manufacturers, contract research organizations, and biotech firms, highlighting their role in driving market expansion and research initiatives. Emerging trends, environmental considerations, regulatory compliance, and competitive strategies are also covered to provide a holistic perspective, enabling stakeholders to identify growth opportunities, optimize operations, and make informed strategic decisions in the Bempedoic Acid API Market.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2024) | USD 120.0 Million |
| Market Revenue (2032) | USD 246.2 Million |
| CAGR (2025–2032) | 9.4% |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User Insights
|
| Key Report Deliverables | Revenue Forecast, Growth Drivers & Restraints, Technology Insights, Market Dynamics, Segmentation Analysis, Regional Insights, Competitive Landscape, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Esperion Therapeutics, Inc., Lonza Group AG, Apotex Inc., Hovione LLC, Zhejiang Hisun Pharmaceutical Co., Ltd., Teva Pharmaceutical Industries Ltd., WuXi AppTec Co., Ltd., Samsung Biologics Co., Ltd., Dr. Reddy’s Laboratories Ltd., Ajanta Pharma Ltd. |
| Customization & Pricing | Available on Request (10% Customization is Free) |
