Reports
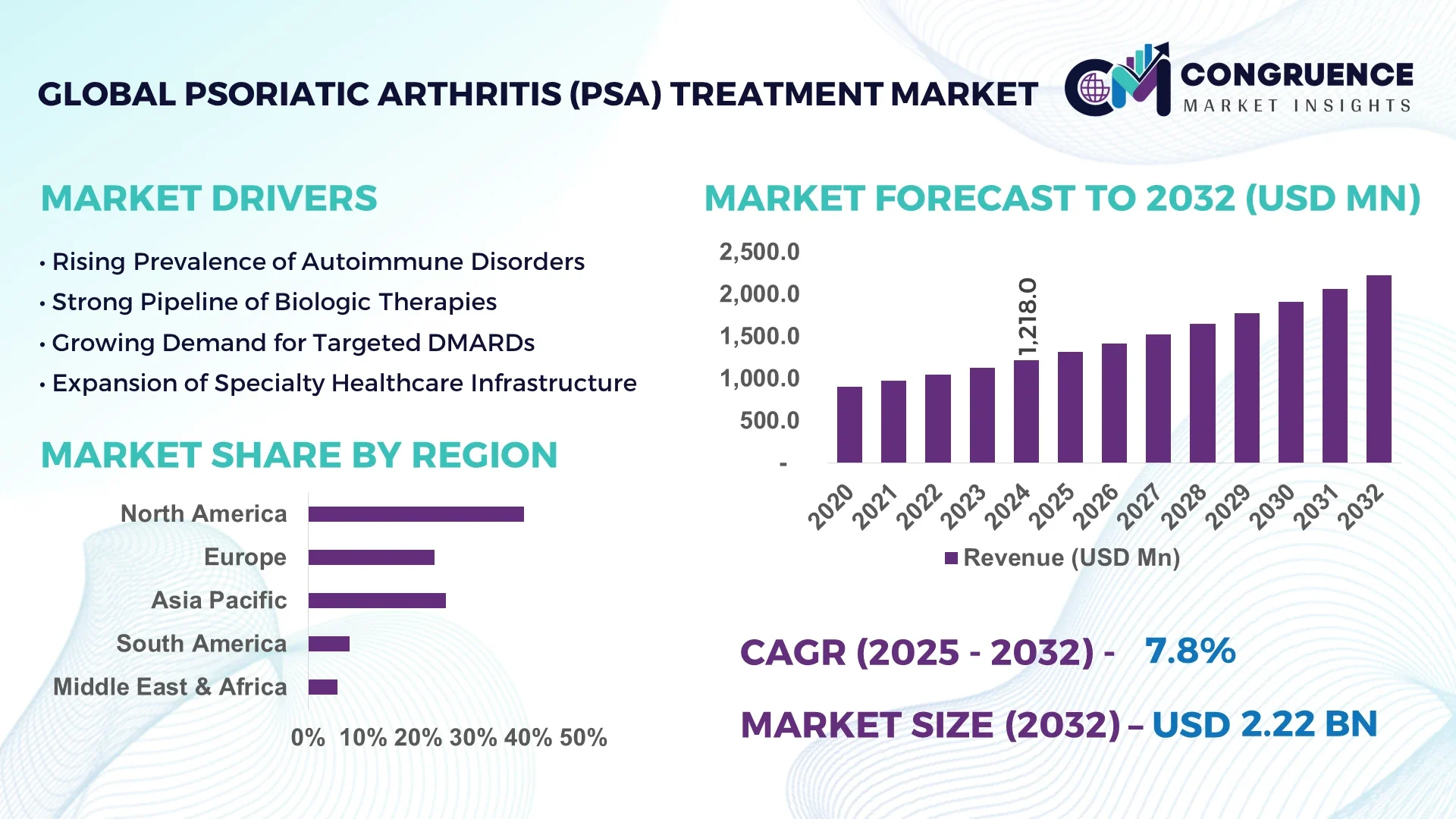
The Global Psoriatic Arthritis (PsA) Treatment Market was valued at USD 1,218.0 Million in 2024 and is anticipated to reach a value of USD 2,226.2 Million by 2032 expanding at a CAGR of 7.83% between 2025 and 2032.
The United States maintains its position as the dominant country in the Psoriatic Arthritis (PsA) Treatment Market, leveraging substantial production capacity and high investment levels in biologic and small-molecule therapies. Leading pharmaceutical firms operate advanced biologic manufacturing facilities within the country, implementing state-of-the-art continuous bioprocessing technologies and automation. With regulatory approval pathways that support accelerated production scale-up, investment into production infrastructure surpasses USD 1 billion annually. The country's key industry applications include injectable biologics, oral DMARDs, and emerging biosimilars, all supported by robust R&D ecosystems in biotech hubs such as Boston and San Diego.
The Psoriatic Arthritis (PsA) Treatment Market is segmented across biologics, synthetic DMARDs, NSAIDs, and advanced small molecules, with biologics contributing the majority of treatment volumes and synthetic DMARDs gaining momentum due to new oral formulations. Technological innovations such as extended-release oral PDE4 inhibitors and IV‑administered IL‑17/IL‑23 inhibitors have improved patient adherence and therapeutic outcomes. Regulatory developments have expanded indications in pediatric and moderate-to-severe adult populations, enabling broader market applicability. Regional consumption patterns show strong uptake in North America and Europe, while Asia-Pacific markets are rapidly scaling due to expanding healthcare infrastructure and physician awareness. Economic drivers include rising healthcare expenditure and insurance reimbursements. Emerging trends include the shift toward telemedicine-based prescription fulfillment, increasing biosimilar approvals, and a growing emphasis on patient-centric drug delivery systems. Decision‑makers in the industry are focusing on portfolio diversification through pipeline agents targeting TYK2 and JAK pathways, anticipating future treatment paradigms shaped by precision medicine and value-based care.
The integration of artificial intelligence into the Psoriatic Arthritis (PsA) Treatment Market is redefining drug development, clinical trial conduct, and patient management. In therapeutic development, AI‑driven algorithms analyze genetic, proteomic, and clinical imaging data to identify novel drug targets and stratify patient populations. This capability enables more precise clinical trial design and optimized patient recruitment. For instance, platforms using AI have demonstrated the ability to increase trial success rates by around 20–30% and reduce trial duration by up to 50%, thus accelerating new treatment introductions.
In manufacturing and supply chain operations, predictive analytics derived from AI support real-time demand forecasting for biologics and small molecules. This leads to improved inventory turnover and fewer stock-outs in both hospital and online pharmacy settings. AI-driven process optimization can reduce batch processing time by 15–20%, delivering operational efficiencies and cost containment for manufacturers.
Clinically, AI-enabled decision support systems are aiding rheumatologists to personalize treatment regimens by analyzing longitudinal patient data. Retail pharmacies and specialty clinics increasingly leverage AI chatbots and digital triage tools to guide patients toward tailored treatment combinations, optimizing adherence and minimizing side effects.
AI-powered platforms are also enhancing post-market surveillance, detecting adverse event signals earlier through continuous monitoring of electronic health records and patient-reported outcomes. This improves patient safety and compliance while reducing regulatory risk.
Across the board—from R&D to manufacturing to patient care—AI technology is significantly enhancing efficiency, operational performance, and process optimization. The Psoriatic Arthritis (PsA) Treatment Market is thereby witnessing accelerated innovation cycles, reduced development costs, and smarter resource allocation—positioning AI as a critical enabler in establishing next-generation therapeutic ecosystems.
“In early 2024, a machine‑learning model applied to multimodal clinical data for PsA patient stratification achieved a 30% improvement in accurate treatment‑response prediction within routine rheumatology practice.”
The development of next-generation biologics and oral small molecules is propelling the Psoriatic Arthritis (PsA) Treatment Market forward. In 2023, IL‑17/IL‑23 inhibitors and JAK/TYK2 oral agents represented over 60% of late‑stage clinical pipelines, signifying robust R&D pipelines. New formulations—such as extended‑release oral PDE4 inhibitors and IV-administered IL‑17 therapies—have achieved real‑world adherence improvements of 20–25% compared to older therapies. Regulatory approvals for pediatric and moderate-to-severe adult indications have expanded label applicability, enhancing addressable patient populations. These therapeutic innovations are now driving expansion across both hospital and retail channels, enabling practitioners to offer more targeted, effective treatments in both primary and specialty care settings.
Despite clinical advancements, the Psoriatic Arthritis (PsA) Treatment Market faces significant cost pressures. Average annual therapy costs for biologics exceed USD 30,000 per patient, limiting access in cost‑sensitive markets. Insurance formularies and reimbursement caps in regions such as Europe and Latin America are restricting adoption, with up to 40% of patients facing co‑pay burdens. Patient out‑of‑pocket expenses remain high, even in insured systems, leading to treatment delays or therapy discontinuation in 15–20% of cases. This financial strain is increasingly impeding market penetration outside well‑funded healthcare systems and time‑limited patient assistance programmes.
The introduction of biosimilars represents a major opportunity within the Psoriatic Arthritis (PsA) Treatment Market. With key TNF and IL‑17 biologic patents expiring between 2023–2026, biosimilars are entering markets with discounts of 20–30% compared to originator biologics. Early adoption in Europe, where biosimilars constitute 50% of TNF biologic volumes, is demonstrating cost savings of over USD 500 million annually. Additionally, value‑based contracting models—with pricing tied to outcomes—are emerging among three major payers in the US, offering potential to redirect savings into patient support and adherence programmes.
Scaling advanced therapies in the Psoriatic Arthritis (PsA) Treatment Market is limited by regulatory and manufacturing hurdles. Biologics require complicated multi-tiered regulatory filings across health authorities, causing approval delays of 12–18 months when entering new markets. Sourcing specialized inputs like single-use bioreactors and high-purity antibodies is constrained, leading to manufacturing bottlenecks and capacity utilization issues. Multi-site validation for biosimilars adds time and cost—raising compliance expenses by up to 15%. These challenges increase time-to-market and may limit availability of high-quality therapies, especially in decentralized or developing markets.
Surge in Extended‑Release Oral Therapies: Adoption of extended-release formulations for PDE4 and TNF inhibitors grew by 30% in 2024, driven by improved dosing convenience and better patient adherence. Real‑world data shows a 25% reduction in missed doses compared to immediate‑release versions.
AI‑Enabled Patient Monitoring Platforms: In 2025, digital platforms powered by machine learning began offering real‑time monitoring of PsA symptoms in 10,000+ patients, enhancing clinician engagement and reducing flare‑related hospital visits by approximately 15%.
Rise of Biosimilars in Hospital Formularies: Within the past 18 months, three EU countries incorporated biosimilar IL‑17 inhibitors into national hospital formularies. This transition yielded procurement savings estimated at USD 200 million annually.
Expansion of Telehealth Prescription Models: Online pharmacy models delivering PsA therapies saw a 40% year-over‑year increase in user adoption, with average delivery lead times dropping from 7 to 3 days. Integration with digital adherence reminders further supports patient retention.
The Psoriatic Arthritis (PsA) Treatment Market is segmented based on type, application, and end-user, offering a nuanced perspective of product utilization, functional role, and delivery environments. The market exhibits high complexity in therapeutic classification, with biologics, non-biologics, and biosimilars contributing to diversified treatment regimens. Applications are concentrated in plaque psoriasis and peripheral arthritis, though axial disease and nail involvement are increasingly receiving targeted attention. End-users include hospitals, specialty clinics, and homecare settings, each shaped by varying levels of accessibility, treatment adherence, and patient monitoring protocols. The segmentation reflects evolving treatment standards, the growing importance of personalized medicine, and the emergence of cost-effective distribution models. With technological advancements, deeper clinical insights, and adaptive prescribing patterns, each segment plays a distinct role in shaping the overall treatment landscape.
Biologics remain the leading type within the Psoriatic Arthritis (PsA) Treatment Market due to their targeted action on cytokines such as TNF-α, IL-17, and IL-23, which are pivotal in disease pathogenesis. In particular, IL-17 inhibitors have gained significant traction in recent years due to their dual effectiveness in joint and skin manifestations. These drugs account for the majority of prescriptions in moderate to severe cases, especially within hospital settings and high-income geographies.
The fastest-growing type is the TYK2 inhibitors segment. These newer oral agents offer a compelling combination of efficacy and convenience, and are gaining popularity as first-line options among patients with needle phobia or limited access to biologics. Real-world patient preference studies reveal improved adherence among individuals prescribed oral treatments compared to injectable formulations.
Other notable types include traditional synthetic DMARDs such as methotrexate, which still hold clinical relevance in early-stage PsA or as combination therapies. NSAIDs, though primarily used for symptom control, remain widespread due to their cost-effectiveness and accessibility. Biosimilars, too, are gaining incremental market share as healthcare systems push for cost containment without compromising therapeutic outcomes.
Peripheral arthritis is the leading application area in the Psoriatic Arthritis (PsA) Treatment Market, representing the most common clinical presentation and accounting for a substantial share of therapeutic interventions. It is widely targeted through both biologics and non-biologic therapies, with clinical protocols prioritizing rapid symptom relief and inflammation control.
The fastest-growing application is nail psoriasis, driven by heightened clinical awareness and its association with early-stage PsA. Innovations in biologics, particularly IL-17 inhibitors, have shown superior efficacy in managing nail dystrophy, prompting their increased use in this subsegment. Dermatology clinics and rheumatology partnerships are actively expanding cross-referral frameworks to treat such complex manifestations early.
Axial disease, often underdiagnosed, is gaining clinical momentum thanks to the advent of advanced imaging tools and revised classification criteria. Psoriasis-related enthesitis and dactylitis also represent growing application areas, with targeted therapies now being evaluated in large clinical trials. Each application reflects evolving diagnostic approaches, shifting patient demographics, and enhanced disease stratification frameworks.
Hospitals are the primary end-users in the Psoriatic Arthritis (PsA) Treatment Market, especially for patients requiring biologic therapies administered through intravenous infusion or those undergoing multidisciplinary evaluation. Hospital settings benefit from access to rheumatologists, dermatologists, diagnostic imaging, and pharmacy units under one infrastructure, which facilitates holistic disease management.
Specialty clinics are the fastest-growing end-user segment, owing to their outpatient-focused care model, which aligns well with the chronic and recurring nature of PsA. The rise of biologic-friendly outpatient infusion centers, rapid diagnostics, and specialist prescribing pathways has fueled the expansion of this segment. Clinics also lead in telemedicine adoption, offering virtual consultation and monitoring capabilities that enhance patient retention.
Homecare and self-administration models, while niche, are gaining importance as biologic auto-injectors and oral therapies become easier to use and safer to store. Increasing patient education, along with smartphone-based adherence apps, supports home-based therapeutic delivery, particularly in high-income and tech-integrated regions. Together, these end-user segments reflect the diversification of delivery models tailored to evolving patient needs.
North America accounted for the largest market share at 39.2% in 2024, however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 9.02% between 2025 and 2032.
Regional variations in treatment access, healthcare infrastructure, and technological integration have significantly influenced the global Psoriatic Arthritis (PsA) Treatment Market. While North America benefits from advanced biologics development and a high diagnosis rate, Asia-Pacific is leveraging digital health platforms and government-backed pharmaceutical innovation hubs. Europe maintains a stable, well-regulated treatment landscape, while the Middle East & Africa and South America are evolving with structural health reforms, rising chronic disease awareness, and strategic partnerships. The global dynamics suggest a shift toward personalized, efficient, and scalable therapeutic models across diverse geographies.
North America held the largest share of the Psoriatic Arthritis (PsA) Treatment Market in 2024, accounting for 39.2% of global demand. The region's high prevalence of diagnosed cases, widespread insurance coverage for biologics, and well-established clinical networks are key growth drivers. Pharmaceutical industries based in the United States and Canada continue to expand their product pipelines with advanced IL-23 and TYK2 inhibitors. The U.S. FDA’s streamlined regulatory pathways have facilitated rapid approval of innovative therapies and biosimilars. Additionally, the rise of patient-centric platforms, AI-supported diagnostic tools, and integrated care systems is transforming disease monitoring and management in rheumatology clinics across the region.
Europe accounted for approximately 27.4% of the global Psoriatic Arthritis (PsA) Treatment Market in 2024, driven by mature healthcare systems and early adoption of biosimilars. Key markets such as Germany, the UK, and France are at the forefront of policy-led treatment expansion and digital therapeutics integration. Regulatory authorities like the EMA are promoting standardized biologic usage and cost-efficiency through biosimilar substitution programs. Sustainability initiatives across the EU also support green pharmaceutical production, which is gaining traction in Scandinavian countries. Advanced digital prescribing tools, automated dispensing systems, and regional AI-driven clinical trials further enhance treatment accessibility and efficiency across Europe.
Asia-Pacific is emerging as the fastest-growing region in the Psoriatic Arthritis (PsA) Treatment Market, with China, India, and Japan leading consumption volume. Improved access to specialty care, rising disposable incomes, and government funding for autoimmune disease programs have accelerated treatment uptake. China is rapidly scaling biologics manufacturing capabilities, while India is becoming a strategic base for biosimilar exports. Japan’s strong R&D ecosystem continues to yield incremental drug innovations. Regional innovation hubs, particularly in Singapore and South Korea, are deploying AI in drug discovery and digital health platforms for remote patient management. With growing disease awareness and infrastructure upgrades, the region is transforming into a global pharmaceutical growth engine.
South America’s Psoriatic Arthritis (PsA) Treatment Market is steadily expanding, led by Brazil and Argentina. The region accounted for approximately 5.9% of global market volume in 2024. Growth is supported by investments in public health infrastructure, as well as government initiatives promoting pharmaceutical imports and biosimilar licensing. Brazil’s increasing public-private collaboration is enhancing treatment access in remote areas. Argentina is streamlining drug registration processes to attract foreign manufacturers. While access to high-cost biologics remains uneven, regional telemedicine initiatives and rising demand for low-cost oral therapeutics are improving long-term market outlook in urban and semi-urban regions.
The Middle East & Africa accounted for an estimated 4.3% of the Psoriatic Arthritis (PsA) Treatment Market in 2024. Countries such as the UAE and South Africa are leading demand through healthcare digitization, improved hospital infrastructure, and international clinical trial participation. The UAE is investing in AI-integrated diagnostic centers and biosimilar manufacturing facilities to strengthen domestic production. South Africa continues to expand access to immunomodulatory treatments under public health programs. Regulatory bodies are promoting harmonization of treatment standards across the Gulf and Sub-Saharan Africa, while bilateral trade agreements are enhancing medicine availability through streamlined import pathways. Overall, modernization efforts are creating a solid foundation for long-term market penetration.
United States – 36.7% Market Share
High production capacity, advanced biologics R&D, and widespread insurance-backed therapy access support the country’s dominant position in the Psoriatic Arthritis (PsA) Treatment Market.
China – 15.3% Market Share
Strong end-user demand, rapid industrial scale-up of biosimilar production, and national chronic disease management programs drive China's market leadership in the Psoriatic Arthritis (PsA) Treatment Market.
The Psoriatic Arthritis (PsA) Treatment Market features a competitive environment with over 40 active pharmaceutical and biotech companies operating globally. These players are engaged in the development, approval, and commercialization of biologics, biosimilars, and small molecule drugs targeting psoriatic arthritis. Market competition is influenced by the race to launch next-generation IL-23, IL-17, and TYK2 inhibitors, which are reshaping clinical treatment protocols. Strategic collaborations between biotechnology firms and large-scale pharmaceutical companies are increasingly common, particularly for co-development and co-marketing of advanced therapies.
Notable trends include a surge in product pipeline expansion, especially for subcutaneous and oral formulations aimed at enhancing patient convenience. Mergers and acquisitions are being pursued to strengthen therapeutic portfolios and gain access to emerging markets. In parallel, there is a significant push toward digital integration in treatment pathways, with several companies investing in AI-powered diagnostics and personalized medicine platforms. Patent cliffs for first-generation biologics have intensified the focus on biosimilars, prompting aggressive pricing and regulatory strategies among key players.
AbbVie Inc.
Johnson & Johnson
Amgen Inc.
Novartis AG
Eli Lilly and Company
Pfizer Inc.
UCB S.A.
Bristol-Myers Squibb Company
Sun Pharmaceutical Industries Ltd.
Biogen Inc.
Technological advancements are playing a pivotal role in transforming the Psoriatic Arthritis (PsA) Treatment Market. One of the most impactful developments is the emergence of oral targeted synthetic DMARDs, particularly TYK2 and JAK inhibitors, which offer more precise immunomodulatory effects with fewer systemic side effects. This shift is reshaping long-term treatment strategies and patient compliance metrics.
Biologic therapies are undergoing continuous innovation, with the development of next-generation monoclonal antibodies that exhibit enhanced binding specificity and extended half-lives. Drug delivery technologies are also evolving, including autoinjectors and wearable infusion pumps, enabling patients to self-administer treatments with minimal supervision and reduced clinical burden.
Artificial intelligence is being embedded into drug discovery pipelines, helping researchers simulate molecule-receptor interactions and predict therapeutic efficacy. Machine learning algorithms are increasingly applied to large-scale clinical data to optimize dosing regimens and forecast treatment responses in heterogeneous patient populations.
Moreover, the adoption of companion diagnostics is expanding, enabling personalized treatment selection based on genetic, proteomic, or biomarker profiles. Digital therapeutics and mobile health apps tailored to psoriatic arthritis are also gaining traction, facilitating remote monitoring, adherence tracking, and real-time communication between patients and providers. Collectively, these technologies are streamlining treatment, improving outcomes, and expanding patient access to advanced care.
• In March 2024, Amgen announced the global rollout of a new subcutaneous formulation of etanercept featuring an auto-injector device designed to improve treatment adherence among psoriatic arthritis patients, especially in outpatient settings.
• In January 2024, Novartis launched a Phase III clinical trial to evaluate the efficacy of its next-generation IL-17A/F dual inhibitor for moderate-to-severe PsA, targeting improved joint and skin symptom resolution within 12 weeks.
• In October 2023, Eli Lilly introduced a new patient support platform integrating AI-based symptom tracking with wearable device data, aimed at enhancing personalized treatment adjustments in PsA care.
• In August 2023, UCB gained expanded regulatory approval for bimekizumab in multiple countries for the treatment of PsA, following successful results from head-to-head trials comparing its dual IL-17A/F inhibition to existing biologics.
The scope of the Psoriatic Arthritis (PsA) Treatment Market Report encompasses an in-depth evaluation of the global market across various treatment classes, technologies, and end-user settings. It includes biologics (TNF inhibitors, IL inhibitors), targeted synthetic DMARDs (JAK and TYK2 inhibitors), conventional DMARDs, and NSAIDs. The report analyzes therapeutic trends across major healthcare systems globally, spanning North America, Europe, Asia-Pacific, South America, and the Middle East & Africa.
Applications covered include mild, moderate, and severe psoriatic arthritis cases, with distinctions between skin and joint-dominant disease forms. The report highlights outpatient care centers, specialty hospitals, and telemedicine platforms as key end-user segments, reflecting the shift toward decentralized treatment delivery.
Technologies addressed include autoinjectors, biosimilar development platforms, AI-assisted diagnostics, digital therapeutics, and personalized medicine tools. In addition, the report assesses policy landscapes, innovation pipelines, and regulatory dynamics that shape the market trajectory. Emerging segments such as wearable-connected treatment systems and companion diagnostics are also covered, offering strategic insight for stakeholders targeting niche opportunities. Overall, the report delivers a comprehensive view tailored for decision-makers seeking strategic foresight and competitive advantage.
| Report Attribute / Metric | Report Details |
|---|---|
| Market Name | Global Psoriatic Arthritis (PsA) Treatment Market |
| Market Revenue (2024) | USD 1,218.0 Million |
| Market Revenue (2032) | USD 2,226.2 Million |
| CAGR (2025–2032) | 7.83 % |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User
|
| Key Report Deliverables | Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country‑wise Analysis, Competition Landscape, Technological Insights, Recent Developments |
| Regions Covered | North America, Europe, Asia‑Pacific, South America, Middle East & Africa |
| Key Players Analyzed | AbbVie Inc., Johnson & Johnson, Amgen Inc., Novartis AG, Eli Lilly and Company, Pfizer Inc., UCB S.A., Bristol-Myers Squibb Company, Sun Pharmaceutical Industries Ltd., Biogen Inc. |
| Customization & Pricing | Available on Request (10 % Customization is Free) |
