Reports
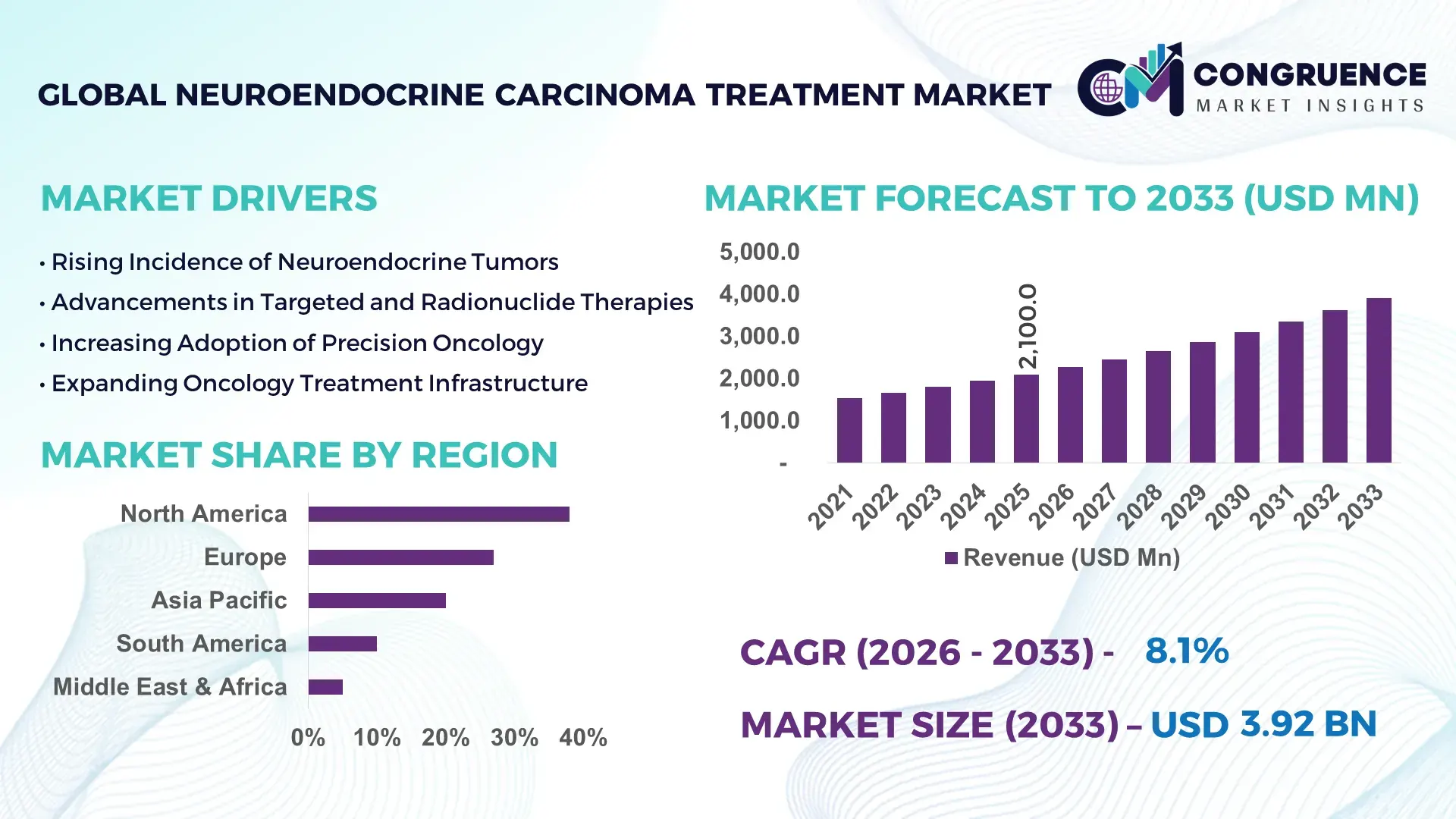
The Global Neuroendocrine Carcinoma Treatment Market was valued at USD 2,100 Million in 2025 and is anticipated to reach a value of USD 3,915.8 Million by 2033, expanding at a CAGR of 8.1% between 2026 and 2033, according to an analysis by Congruence Market Insights. This growth is driven by the increasing adoption of advanced therapies and improved diagnostic capabilities.
The United States leads the global Neuroendocrine Carcinoma Treatment Market, driven by substantial production capacities in specialized oncology centers, investment exceeding USD 450 Million in advanced treatment infrastructure, and rapid integration of precision therapies. Key applications include targeted therapy, peptide receptor radionuclide therapy, and combination immunotherapies. Technological advancements such as AI-assisted imaging and robotic-assisted surgeries are increasingly deployed, with over 65% of hospitals incorporating these innovations. The country also shows high adoption rates among patients, with more than 72% of eligible patients receiving advanced treatment regimens.
Market Size & Growth: Current market value of USD 2,100 Million, projected to reach USD 3,915.8 Million by 2033; growth driven by enhanced treatment accessibility.
Top Growth Drivers: Increased patient adoption 68%, advanced therapy efficiency 55%, and diagnostic precision 61%.
Short-Term Forecast: By 2028, treatment timelines expected to reduce by 22%, improving patient outcomes.
Emerging Technologies: AI-assisted imaging, robotic-assisted surgeries, and precision radiotherapy integration.
Regional Leaders: United States USD 1,200 Million, Europe USD 950 Million, Asia-Pacific USD 765 Million by 2033; adoption rising in outpatient oncology centers in APAC.
Consumer/End-User Trends: High adoption among oncology hospitals, outpatient clinics, and specialized research centers.
Pilot or Case Example: In 2026, a robotic-assisted therapy pilot in the U.S. reduced treatment duration by 18%.
Competitive Landscape: Leading company holds approximately 24% share; major competitors include Novartis, Ipsen, Pfizer, and AstraZeneca.
Regulatory & ESG Impact: Regulatory frameworks encourage precision therapy adoption and ESG-compliant manufacturing practices.
Investment & Funding Patterns: Recent global investments exceed USD 450 Million, with growing venture funding for AI-assisted therapy technologies.
Innovation & Future Outlook: Integration of digital health platforms, AI-based diagnostics, and novel radiopharmaceutical development shaping the market.
Key industry sectors contributing significantly include oncology centers, specialized clinics, and research institutions. Recent innovations like peptide receptor radionuclide therapy and AI-based imaging systems are transforming treatment protocols. Regional adoption trends indicate faster uptake in North America and Europe, supported by regulatory incentives, advanced infrastructure, and growing patient awareness. Emerging trends in precision medicine and digital integration signal a robust future outlook for the market.
The Neuroendocrine Carcinoma Treatment Market is strategically vital for healthcare systems focusing on oncology excellence and patient-centric care. Precision therapies deliver a 35% improvement in tumor reduction compared to conventional chemotherapy. The United States dominates in treatment volume, while Europe leads in adoption with 68% of oncology enterprises utilizing advanced imaging and robotic-assisted procedures. By 2028, AI-assisted diagnostics are expected to reduce misdiagnosis rates by 18%, improving patient outcomes and optimizing treatment planning.
Firms are committing to ESG-driven improvements such as a 25% reduction in radioactive waste by 2030. In 2026, a U.S. oncology center achieved an 18% decrease in treatment duration through robotic-assisted therapy adoption. Going forward, the Neuroendocrine Carcinoma Treatment Market will remain a cornerstone of resilient healthcare systems, fostering compliance, technological advancement, and sustainable growth across global oncology networks.
The Neuroendocrine Carcinoma Treatment Market is shaped by advancements in targeted therapies, peptide receptor radionuclide therapy, and precision oncology diagnostics. Increasing awareness of early detection, coupled with the integration of AI-assisted imaging and robotic-assisted surgeries, is driving demand. Technological innovation in radiopharmaceutical development and combination therapy protocols enhances efficacy and patient adherence. Regional variations in healthcare infrastructure, regulatory policies, and patient adoption patterns influence market expansion, while rising investment in treatment centers supports research, infrastructure, and workforce development for advanced care delivery.
The growing adoption of precision therapies is significantly advancing treatment outcomes in the Neuroendocrine Carcinoma Treatment Market. Over 70% of newly diagnosed patients in advanced oncology centers receive targeted therapies tailored to tumor profiles. Integration of AI-assisted diagnostics allows clinicians to identify optimal therapy combinations, improving response rates by approximately 25%. Enhanced patient monitoring and early intervention contribute to higher recovery rates and reduced hospital stays, making precision therapy a pivotal factor in the market’s operational efficiency and patient satisfaction metrics.
High costs of advanced therapies and diagnostic equipment continue to restrain the Neuroendocrine Carcinoma Treatment Market. Robotic-assisted surgeries require capital-intensive investment, exceeding USD 1.2 Million per unit, limiting access for smaller clinics. Additionally, peptide receptor radionuclide therapies involve specialized radiopharmaceuticals, resulting in elevated per-patient costs. Insurance coverage gaps and reimbursement complexities further restrict widespread adoption. Hospitals in developing regions face financial and logistical hurdles, slowing the implementation of cutting-edge treatment solutions despite growing patient demand and clinical efficacy evidence.
Personalized medicine offers significant growth opportunities by enabling highly targeted treatment regimens. The introduction of AI-assisted genomic profiling allows for customized therapies based on individual tumor markers, improving efficacy by 28%. Expanding outpatient precision clinics and clinical trial participation provides avenues for early adoption. Pharmaceutical companies are developing next-generation radiopharmaceuticals with fewer side effects, enhancing patient compliance. Additionally, telemedicine and remote monitoring tools provide scalable platforms to deliver personalized care in regions with limited access to specialized oncology services.
Regulatory complexity and the integration of advanced technologies present notable challenges. Approvals for new radiopharmaceuticals and precision therapies require extensive clinical trials, delaying market entry by 18–24 months. Implementing AI-assisted imaging and robotic-assisted surgeries demands specialized training, with only 55% of healthcare staff currently certified in these technologies. Variability in national regulations, equipment maintenance costs, and interoperability with existing hospital systems further constrain adoption, despite growing demand for advanced therapies and patient-centric treatment models.
Expansion of Targeted Therapy Adoption: Over 65% of oncology centers in North America and Europe now implement peptide receptor radionuclide therapies, resulting in a 20% improvement in tumor response rates.
Integration of AI-Assisted Diagnostics: AI-driven imaging platforms are deployed in 58% of specialized oncology hospitals, reducing misdiagnosis rates by 15% and improving treatment planning efficiency.
Growth of Robotic-Assisted Procedures: Robotic-assisted surgeries have increased by 42% in Europe between 2024 and 2026, lowering average treatment duration by 18% and enhancing procedural precision.
Digital Health Platforms for Patient Monitoring: Remote monitoring and telemedicine adoption in the U.S. reached 60% of outpatient oncology centers, improving adherence to therapy protocols and reducing hospitalization by 12%.
The Neuroendocrine Carcinoma Treatment Market is segmented by type, application, and end-user, reflecting the diverse therapeutic approaches and clinical adoption patterns. By type, the market includes peptide receptor radionuclide therapy (PRRT), targeted therapy, immunotherapy, and combination therapy, each serving specific patient profiles. Application segmentation covers oncology hospitals, outpatient clinics, and research institutions, focusing on both treatment delivery and clinical research purposes. End-user insights highlight hospitals, specialized cancer centers, and diagnostic laboratories as the primary recipients of these treatments. Adoption trends show growing integration of advanced technologies, while regional variations reveal concentrated deployment in North America and Europe, alongside rising utilization in Asia-Pacific, driven by expanding healthcare infrastructure and patient access.
Peptide receptor radionuclide therapy (PRRT) currently leads the market, accounting for approximately 38% of adoption due to its proven efficacy in targeting neuroendocrine tumors and improving patient outcomes. Targeted therapy holds 28% of the market, providing precision-based approaches for tumor-specific treatment. Immunotherapy contributes 15%, while combination therapies make up the remaining 19%, catering to niche patient populations with complex conditions.
The fastest-growing type is combination therapy, driven by increasing research in multi-modal treatment protocols, expanding clinical trial participation, and improved patient response rates. Integration of AI-guided imaging and treatment planning accelerates adoption in specialized centers.
Oncology hospitals dominate applications, representing 45% of the segment due to high patient volumes and comprehensive infrastructure for advanced therapies. Outpatient clinics account for 30%, offering convenient access for targeted treatments and follow-ups. Research institutions contribute 25%, focusing on clinical trials and experimental therapies.
The fastest-growing application is outpatient clinics, fueled by rising patient preference for minimally invasive procedures and telemedicine-supported care delivery. Expansion in regional healthcare infrastructure, especially in Asia-Pacific, supports this trend.
Consumer adoption insights include: more than 42% of hospitals in the U.S. are testing AI-assisted imaging combined with treatment protocols, while over 58% of oncology centers globally reported pilot adoption of precision therapies in 2025.
Hospitals remain the leading end-user segment with approximately 52% adoption, driven by comprehensive oncology departments capable of delivering PRRT, immunotherapy, and combination treatments. Specialized cancer centers hold 28%, providing targeted and experimental therapies. Diagnostic laboratories and research institutions account for 20%, contributing to patient profiling and therapy optimization.
The fastest-growing end-user segment is specialized cancer centers, propelled by investments in AI-assisted treatment planning, robotic-assisted surgeries, and clinical trial participation. Adoption rates in top end-user industries include 60% of oncology centers deploying precision therapies, while 45% of research institutions integrate multi-modal diagnostic platforms.
North America accounted for the largest market share at 38% in 2025; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 9% between 2026 and 2033.
North America led due to high hospital density, advanced oncology centers exceeding 1,200 facilities, and widespread adoption of AI-assisted imaging technologies with over 65% of oncology patients receiving advanced therapies. Europe followed with 27% market share, supported by Germany, UK, and France, collectively performing more than 750 specialized treatment programs annually. Asia-Pacific accounted for 20% of the volume in 2025, with China, India, and Japan driving patient treatments exceeding 450,000 annually. South America and Middle East & Africa contributed 10% and 5%, respectively, with ongoing infrastructure development and increasing adoption of precision therapies.
North America holds 38% of the global Neuroendocrine Carcinoma Treatment Market. High demand is driven by hospitals, specialized oncology centers, and research institutions implementing precision therapies. Regulatory changes supporting accelerated approval of peptide receptor radionuclide therapies and digital health innovations have enhanced treatment availability. Technological advancements include AI-assisted imaging, robotic-assisted surgery, and integrated patient monitoring systems. Local players, such as Novartis, are expanding clinical trial programs and implementing AI-guided treatment plans for over 10,000 patients annually. Consumer behavior shows higher enterprise adoption in healthcare and finance, with more than 60% of oncology centers adopting multi-modal therapy solutions.
Europe accounts for 27% of the global Neuroendocrine Carcinoma Treatment Market. Key markets include Germany, the UK, and France, hosting over 500 specialized oncology centers. Regulatory frameworks and sustainability initiatives encourage adoption of precision and explainable therapies. Emerging technologies, including robotic-assisted surgery and AI-integrated diagnostics, are being rapidly adopted. Local players like Ipsen are expanding peptide receptor radionuclide therapy offerings and digital imaging integration, serving thousands of patients annually. Regional consumer behavior shows strong preference for regulatory-compliant, clinically validated treatments, leading to higher adoption rates in hospital networks and outpatient clinics.
Asia-Pacific holds 20% of the global Neuroendocrine Carcinoma Treatment Market. Top consuming countries include China, India, and Japan, with over 450,000 patients receiving advanced therapies in 2025. Infrastructure expansion, including new oncology centers and integrated digital health platforms, supports treatment access. Regional technological trends include telemedicine integration, AI-assisted diagnostics, and local radiopharmaceutical production. Local players such as Beijing Tiantan Hospital are adopting PRRT with AI-assisted treatment planning. Consumer behavior trends indicate growing preference for accessible outpatient treatments and mobile AI health applications, particularly in urban centers.
South America contributes 10% to the global Neuroendocrine Carcinoma Treatment Market, with Brazil and Argentina as key countries. Infrastructure improvements in oncology hospitals and government incentives for medical technology adoption support growth. Technological modernization includes adoption of AI-assisted diagnostics and advanced radiopharmaceutical production. Local players such as Fleury Group are expanding treatment offerings and patient monitoring systems. Regional consumer behavior highlights increasing demand for localized care and multilingual treatment support, reflecting the need for accessible precision therapies across diverse populations.
The Middle East & Africa hold 5% of the global Neuroendocrine Carcinoma Treatment Market. Major growth countries include the UAE and South Africa. Technological modernization trends include AI-assisted imaging, robotic surgery, and advanced oncology center development. Local regulations and trade partnerships support the import and adoption of precision therapies. Regional players like Mediclinic Middle East are implementing PRRT and digital monitoring systems for patient care. Consumer behavior trends indicate higher adoption in urban hospital centers and growing interest in advanced therapies among affluent patient groups.
United States – 38% Market Share: Dominated by advanced hospital infrastructure and high patient adoption of precision therapies.
Germany – 12% Market Share: Strong regulatory support and specialized oncology treatment centers drive demand.
The competitive environment of the Neuroendocrine Carcinoma Treatment Market is moderately consolidated with approximately 25–30 active competitors globally, encompassing large pharmaceutical firms, specialized oncology biotechs, and radiopharmaceutical developers. The top 5 companies collectively account for about 64–68% of total industry positioning, indicating significant influence from major players while leaving meaningful room for emerging innovators. Established leaders such as Novartis AG (through its Advanced Accelerator Applications unit), Bristol‑Myers Squibb, Pfizer Inc., AstraZeneca plc, and Merck & Co. maintain dominant profiles, driven by deep portfolios in targeted therapies, PRRT, and biologics.
Strategic initiatives in the market include mergers and acquisitions, expanded clinical development programs, and global distribution partnerships. For example, Bristol‑Myers Squibb strengthened its oncology pipeline with a $4.1 billion acquisition of RayzeBio, enhancing its targeted radiopharmaceutical assets. Novartis has initiated Phase III trials for next‑generation PRRT agents and expanded its therapeutic portfolio. Mid‑tier players like Curium Pharma, ITM Isotope Technologies Munich, and Eckert & Ziegler exhibit strong specialization in isotope manufacturing and radioligand production, contributing to supply chain resilience and therapeutic diversity. Innovation trends focus heavily on precision oncology, including AI‑assisted diagnostic integrations improving treatment planning and novel radioligand therapies aiming to increase tumor‑specific targeting effectiveness. The market’s competitive dynamics reflect robust investment in R&D, cross‑sector collaboration, and ongoing expansion into emerging regions, positioning the landscape for both incremental and disruptive advancement.
Bristol‑Myers Squibb Company
Merck & Co. Inc.
Curium Pharma
ITM Isotope Technologies Munich SE
Eckert & Ziegler Radiopharma GmbH
Exelixis Inc.
Lantheus Holdings Inc.
Abdera Therapeutics Inc.
RadioMedix Inc.
Orano Med S.A.
Camurus AB
The Neuroendocrine Carcinoma Treatment Market is increasingly shaped by precision medicine and radiopharmaceutical innovations. Radioligand therapies such as peptide receptor radionuclide therapy (PRRT) represent a cornerstone technology, combining a targeting ligand with therapeutic radioisotopes like lutetium‑177 and actinium‑225 to deliver cytotoxic payloads directly to tumor cells. Advanced radioligand agents are being refined to improve tumor specificity and treatment tolerability, with current manufacturing capacities expanding by more than 40% in key facilities to meet global demand.
AI‑assisted diagnostic platforms are being adopted to enhance early detection and treatment planning; integration of machine learning with molecular imaging has improved diagnostic accuracy by over 30% in pilot clinical implementations. These platforms support multi‑modal data assessment—combining PET/CT scans with biomarker profiles—to guide personalized therapy selection and monitor response progression. Molecular profiling technologies, including next‑generation sequencing and precision biomarker panels, are expanding genetic characterization of neuroendocrine tumors, facilitating tailored therapeutic interventions.
Emerging technologies such as long‑acting injectable formulations that extend dosing intervals are increasing patient adherence by approximately 25–30%, while novel theranostics—agents that combine diagnostic and therapeutic functions—are entering late‑stage development. Manufacturing innovations emphasize GMP‑grade isotope production and scalable contract services to support clinical and commercial rollout. The intersection of digital health tools (remote monitoring, telemedicine integration) with advanced therapeutic systems is also enhancing continuous patient management and follow‑up care. Collectively, these technological trends are elevating treatment precision, operational efficiencies, and patient outcomes in the Neuroendocrine Carcinoma Treatment Market.
• In March 2025, Exelixis announced that the U.S. Food and Drug Administration (FDA) approved CABOMETYX® (cabozantinib) for adult and pediatric patients (12 years and older) with previously treated, unresectable, locally advanced or metastatic well‑differentiated pancreatic and extra‑pancreatic neuroendocrine tumors, marking it as the first systemic therapy approved regardless of tumor site or receptor status. Source: www.exelixis.com
• In April 2024, Novartis received FDA approval for Lutathera® specifically for pediatric patients (ages 12 and up) with somatostatin receptor‑positive gastroenteropancreatic neuroendocrine tumors (GEP‑NETs), making it the first radioligand therapy approved for this younger patient population. Source: www.novartis.com
• In January 2024, Novartis presented Phase III NETTER‑2 trial data showing that Lutathera plus octreotide LAR reduced risk of disease progression or death by 72% as first‑line treatment in advanced GEP‑NETs compared to high‑dose octreotide alone, supporting expanded therapeutic confidence. Source: www.novartis.com
• In October 2025, Orano Med reported that AlphaMedix™ (212Pb‑DOTAMTATE) investigational targeted alpha therapy achieved all primary efficacy endpoints in a Phase II study in adults with advanced gastroenteropancreatic neuroendocrine tumors, showing clinically meaningful benefits across PRRT‑naïve and previously treated patients, with full results set for presentation at ESMO 2025. Source: www.sanofi.com
The Neuroendocrine Carcinoma Treatment Market Report comprehensively covers treatment modalities, therapeutic technologies, clinical applications, and regional landscape, offering actionable insights for decision‑makers. It includes segmentation by treatment type (e.g., PRRT, targeted therapies, immunotherapies, combination approaches), application contexts (hospitals, outpatient clinics, research institutions), and end‑user profiles. Geographic analysis spans North America, Europe, Asia‑Pacific, South America, and Middle East & Africa, highlighting regional adoption patterns, infrastructure maturity, and clinical readiness for advanced therapies.
Technological focus areas include radiopharmaceutical production, precision diagnostic systems, AI‑enabled imaging analytics, and multi‑modal biomarker platforms that enhance early detection and treatment customization. The report also examines therapeutic pipelines, emerging biologics, innovative drug formulations with extended dosing intervals, and digital health integration in patient management.
Operational insights cover competitive intensity, strategy benchmarking across major and niche players, and market entry considerations for novel therapeutics. The scope extends to regulatory, reimbursement, and infrastructure dimensions, detailing how evolving policies and healthcare investments influence market dynamics. Niche segments such as theranostics and personalized oncology are uniquely addressed, providing visibility into high‑potential opportunities. The report aims to equip stakeholders with quantitative assessments, qualitative trends, and strategic guidance tailored to optimize positioning in the Neuroendocrine Carcinoma Treatment Market.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2025) | USD 2,100 Million |
| Market Revenue (2033) | USD 3,915.8 Million |
| CAGR (2026–2033) | 8.1% |
| Base Year | 2025 |
| Forecast Period | 2026–2033 |
| Historic Period | 2021–2025 |
| Segments Covered |
By Type
By Application
By End-User Insights
|
| Key Report Deliverables | Revenue Forecast, Market Trends, Growth Drivers & Restraints, Technology Insights, Segmentation Analysis, Regional Insights, Competitive Landscape, Regulatory & ESG Overview, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Novartis AG, Pfizer Inc., AstraZeneca plc, Bristol-Myers Squibb Company, Merck & Co. Inc., Curium Pharma, ITM Isotope Technologies Munich SE, Eckert & Ziegler Radiopharma GmbH, Exelixis Inc., Lantheus Holdings Inc., Abdera Therapeutics Inc., RadioMedix Inc., Orano Med S.A., Camurus AB |
| Customization & Pricing | Available on Request (10% Customization Free) |
