Reports
The Global Clinical Trial Management System Market is expected to expand at a CAGR of 10.3% between 2023 and 2030. The Clinical Trial Management System (CTMS) market boasts a dynamic landscape driven by the increasing complexity of clinical trials and the growing demand for streamlined research processes. CTMS platforms serve as pivotal tools in managing various aspects of clinical trials, including protocol design, participant recruitment, data collection, and regulatory compliance. With the rise in outsourcing of clinical trials and the adoption of digital technologies, the market witnesses a surge in cloud-based CTMS solutions and integrated platforms that offer real-time data access and collaboration capabilities. These advancements underscore the pivotal role of CTMS in enhancing efficiency, transparency, and compliance across the clinical trial lifecycle.
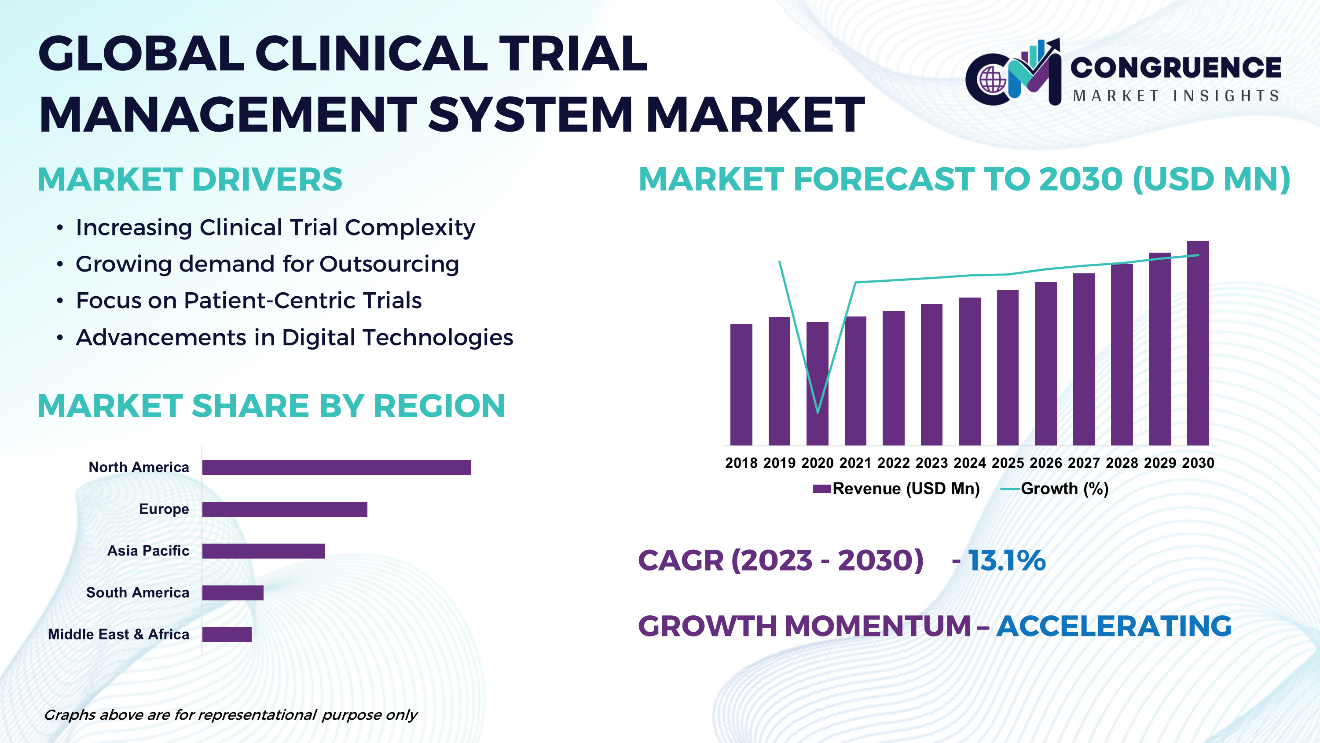
Clinical Trial Management System Market Major Driving Forces
Increasing Clinical Trial Complexity: Rising complexity in clinical trial design, including larger participant pools, diverse study endpoints, and stringent regulatory requirements, drives the demand for CTMS platforms to streamline trial management processes.
Growing demand for Outsourcing: Pharmaceutical companies and contract research organizations (CROs) increasingly outsource clinical trial activities, fueling the need for centralized, efficient CTMS solutions to manage multi-site collaborations and ensure data integrity.
Focus on Patient-Centric Trials: Shift towards patient-centric trial designs, with an emphasis on remote monitoring, decentralized trials, and patient engagement, drives the adoption of CTMS solutions that support remote data collection and participant interaction.
Advancements in Digital Technologies: Technological advancements, including cloud computing, artificial intelligence, and data analytics, enable the development of more sophisticated CTMS platforms with enhanced functionalities for data management, analytics, and real-time reporting.
Clinical Trial Management System Market Key Opportunities
Integration of Artificial Intelligence (AI) and Machine Learning: Leveraging AI and machine learning algorithms within CTMS platforms presents an opportunity to automate repetitive tasks, analyze vast amounts of data for insights, and optimize trial protocols for enhanced efficiency and efficacy.
Expansion of Decentralized Trial Capabilities: With the shift towards decentralized clinical trials, there's an opportunity to develop CTMS solutions that support remote monitoring, wearable device integration, and virtual participant engagement, thereby improving patient recruitment, retention, and data quality.
Enhanced Regulatory Compliance Solutions: Developing CTMS platforms with robust regulatory compliance features, including electronic data capture (EDC), audit trail functionality, and integration with regulatory reporting systems, offers an opportunity to streamline regulatory submissions, minimize compliance risks, and expedite trial approval processes.
Clinical Trial Management System Market Key Trends
· Increasing adoption of RBM strategies within CTMS platforms to prioritize resources, detect risks early, and ensure data quality in clinical trials.
· Growing trend of integrating blockchain technology into CTMS solutions to enhance data security, transparency, and integrity throughout the trial lifecycle.
· Rise of mobile CTMS applications offering on-the-go access to trial data, participant information, and task management, improving operational efficiency and flexibility for trial teams.
· Focus on interoperability between CTMS platforms and EHR systems to streamline data exchange, enhance data accuracy, and facilitate seamless integration of patient health records into clinical trials.
· Increasing emphasis on patient-centric trial designs, driving the development of CTMS solutions with patient engagement features, remote monitoring capabilities, and user-friendly interfaces to enhance participant experience and retention.
· Growing demand for CTMS platforms with advanced analytics and reporting functionalities, enabling real-time data insights, trend analysis, and customizable reporting to support informed decision-making and trial optimization.
· Integration of AI and predictive analytics into CTMS platforms to forecast trial outcomes, identify potential risks, and optimize resource allocation, improving trial efficiency and success rates.
Market Competition Landscape
The competitive landscape of the Clinical Trial Management System (CTMS) market is characterized by intense rivalry among industry players, driven by factors such as technological innovation, market expansion strategies, and regulatory compliance. Companies vie for market share through product differentiation, strategic partnerships, and mergers and acquisitions, aiming to establish themselves as leading providers of comprehensive CTMS solutions tailored to the evolving needs of clinical research organizations.
Key players in the global Clinical Trial Management System market implement various organic and inorganic strategies to strengthen and improve their market positioning. Prominent players in the market include:
· Oracle
· Dassault Systèmes
· International Business Machines Corporation (IBM)
· Parexel International Corporation
· IQVIA Holdings Inc.
· Veeva Systems Inc.
· eClinical Solutions LLC
· ArisGlobal LLC
· OmniComm Systems, Inc.
· Bioclinica
· Bio-Optronics, Inc.
· DSG, Inc.
· MedNet Solutions, Inc.
· MasterControl, Inc.
· Forte Research Systems, Inc.
· OpenClinica, LLC
· Clinical Ink, Inc.
· Medidata Solutions Japan, Ltd.
|
Report Attribute/Metric |
Details |
|
Base Year |
2022 |
|
Forecast Period |
2023 – 2030 |
|
Historical Data |
2018 to 2022 |
|
Forecast Unit |
Value (US$ Mn) |
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Segments Covered |
· By Type (Enterprise CTMS, and Site CTMS) · By Deployment Model (Software, and Service) · By End-User (Pharmaceutical and Biotechnology Companies, Contract Research Organizations (CROs), Medical Device Companies, and Academic Research Institutes) |
|
Geographies Covered |
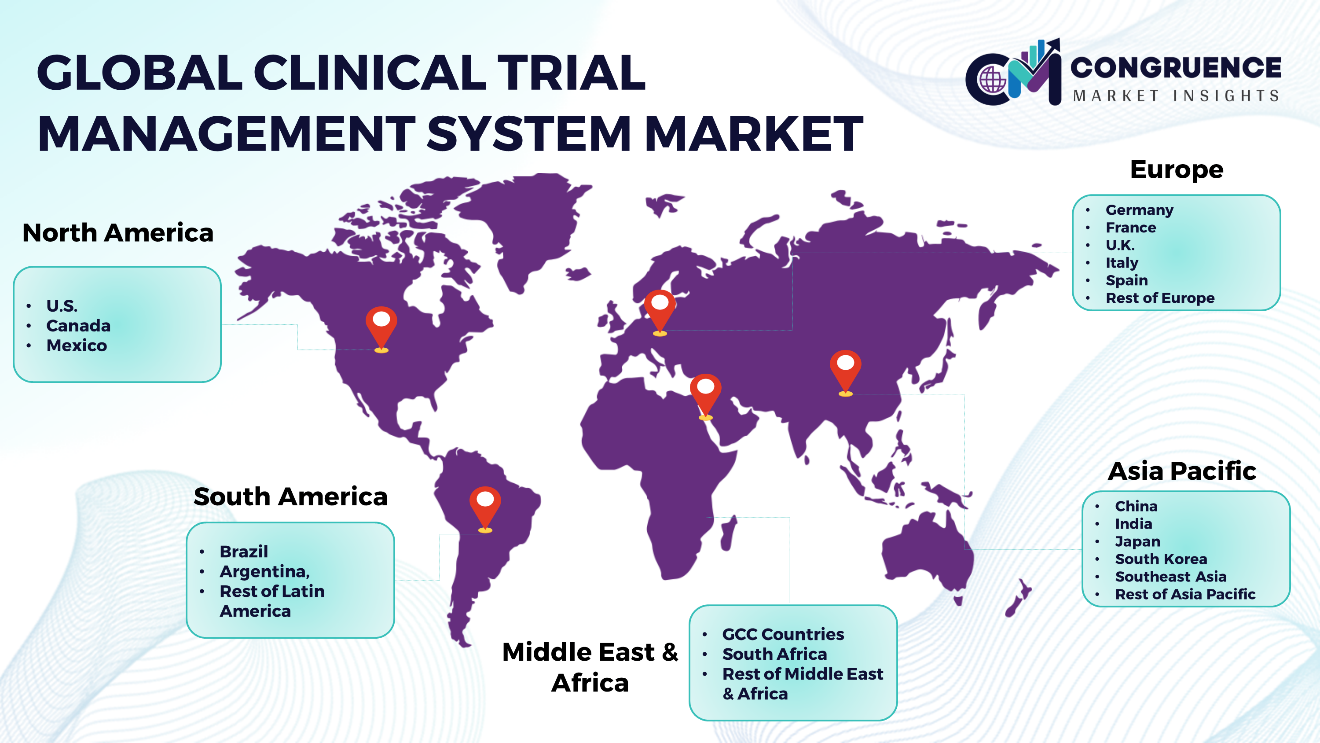
North America: U.S., Canada and Mexico Europe: Germany, France, U.K., Italy, Spain, and Rest of Europe Asia Pacific: China, India, Japan, South Korea, Southeast Asia, and Rest of Asia Pacific South America: Brazil, Argentina, and Rest of Latin America Middle East & Africa: GCC Countries, South Africa, and Rest of Middle East & Africa |
|
Key Players Analyzed |
Oracle, Dassault Systèmes, International Business Machines Corporation (IBM), Parexel International Corporation, IQVIA Holdings Inc., Veeva Systems Inc., eClinical Solutions LLC, ArisGlobal LLC, OmniComm Systems, Inc., Bioclinica, Bio-Optronics, Inc., DSG, Inc., MedNet Solutions, Inc., MasterControl, Inc., Forte Research Systems, Inc., OpenClinica, LLC, Clinical Ink, Inc., and Medidata Solutions Japan, Ltd. |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
