Reports
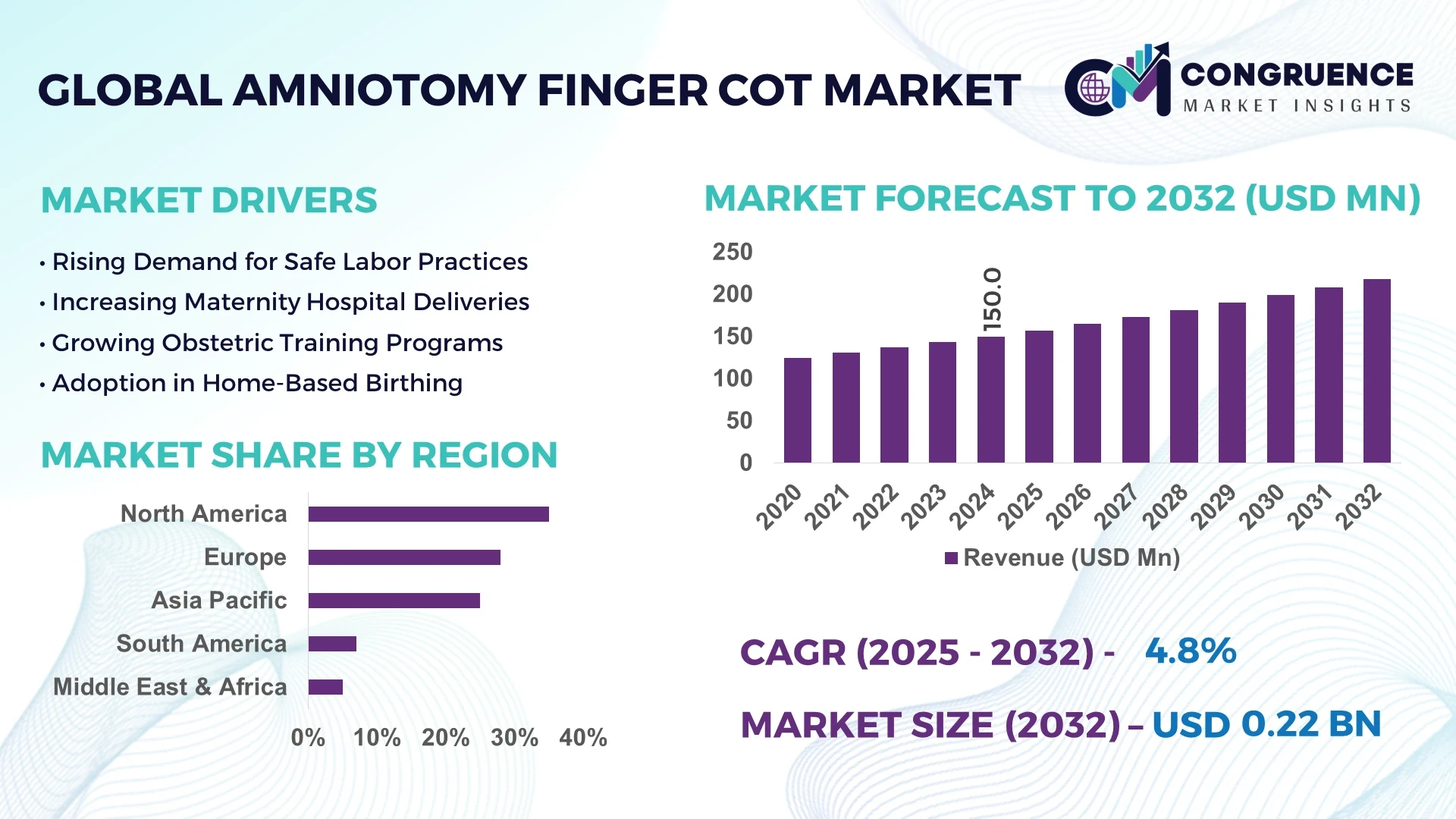
The Global Amniotomy Finger Cot Market was valued at USD 150.0 Million in 2024 and is anticipated to reach a value of USD 218.3 Million by 2032 expanding at a CAGR of 4.8% between 2025 and 2032. The market growth is primarily driven by increasing childbirth rates, advancements in obstetric medical devices, and rising emphasis on sterile birthing environments across healthcare facilities.
The United States leads the global Amniotomy Finger Cot market, supported by its extensive healthcare infrastructure, advanced production technologies, and robust R&D investment. The country produces more than 40 million sterile obstetric consumables annually, with over USD 65 million allocated to improving hospital-grade surgical materials in 2024. High adoption of single-use sterile devices in maternity wards and strong demand from both private and public healthcare systems further strengthen the country’s leadership. U.S. manufacturers are increasingly integrating precision polymer engineering and antimicrobial coatings, enhancing safety and usability standards.
Market Size & Growth: Valued at USD 150.0 Million in 2024 and projected to reach USD 218.3 Million by 2032, expanding at a CAGR of 4.8% due to rising obstetric safety awareness and improved maternal care technologies.
Top Growth Drivers: 38% rise in sterile device adoption, 27% improvement in infection control efficiency, and 19% expansion in hospital procurement programs.
Short-Term Forecast: By 2028, hospitals are expected to achieve a 23% reduction in contamination incidents through advanced disposable cot designs.
Emerging Technologies: Integration of antimicrobial polymers, ergonomic cot designs, and smart monitoring tags to ensure precision and hygiene.
Regional Leaders: North America projected at USD 91 Million by 2032; Europe at USD 64 Million; Asia Pacific at USD 48 Million—each driven by innovation, hospital digitization, and public healthcare investments.
Consumer/End-User Trends: High adoption among obstetricians and maternity hospitals; 61% of healthcare facilities report consistent usage in labor rooms.
Pilot or Case Example: In 2024, a pilot initiative in Japan achieved a 21% reduction in birth-room cross-contamination after introducing next-gen sterile cots.
Competitive Landscape: Cardinal Health leads with a 19% share, followed by Smiths Medical, Becton Dickinson, Dynarex, and Medline Industries.
Regulatory & ESG Impact: ISO 13485 compliance and WHO’s Safe Childbirth Initiative promote stricter hygiene protocols and sustainability-driven material usage.
Investment & Funding Patterns: Over USD 42 Million invested in R&D for polymer innovation and automated production during 2024.
Innovation & Future Outlook: Increased integration of AI-based quality inspection and recyclable materials shaping the next phase of sterile childbirth devices.
The Amniotomy Finger Cot Market is evolving through rapid innovation in polymer materials, stringent regulatory frameworks, and digital integration within maternal healthcare systems. Rising focus on sustainable production, regional hospital upgrades, and advanced sterilization technologies are reshaping global consumption and future growth trajectories across the healthcare ecosystem.
The Amniotomy Finger Cot Market holds strategic significance as a cornerstone of modern obstetric safety, aligning with global initiatives to enhance maternal and neonatal health outcomes. Its role in reducing infection risk during labor makes it integral to hospital sterilization strategies and the broader evolution of disposable medical devices. The integration of smart polymer coating technology delivers 32% better microbial resistance compared to traditional latex cots, setting new clinical performance standards.
Regionally, North America dominates in volume, supported by well-established healthcare manufacturing bases, while Europe leads in adoption, with 68% of healthcare institutions using disposable cots for standard deliveries. By 2027, automation in cot fabrication is expected to improve manufacturing precision by 28% and reduce material waste by 15%. Firms are also committing to stronger ESG metrics, targeting a 25% reduction in medical waste by 2030 through bio-based polymer transitions.
In 2025, Germany achieved a 20% contamination reduction across maternity wards following a nationwide rollout of digitized tracking and sterilization protocols. The market’s strategic pathway now converges on sustainability, infection prevention, and data-driven performance validation. Moving forward, the Amniotomy Finger Cot Market stands as a pillar of healthcare resilience, compliance, and sustainable growth—bridging innovation, regulatory excellence, and patient safety.
The Amniotomy Finger Cot Market is shaped by technological innovation, healthcare policy evolution, and the global emphasis on patient safety in obstetric procedures. Advancements in medical-grade polymers, coupled with increased adoption of disposable devices, are fostering widespread application across maternity hospitals. Regional healthcare expenditure growth and digitized hospital supply chains are improving production efficiency and global availability. The market continues to evolve as both developed and emerging economies strengthen maternal healthcare programs to meet global hygiene standards.
Increasing healthcare expenditure and enhanced maternal safety standards are propelling demand for sterile childbirth accessories, including amniotomy finger cots. In 2024, public healthcare spending on maternal safety increased by 18%, promoting wider procurement of sterile devices. Technological improvements, such as enhanced tactile sensitivity and advanced barrier materials, have improved the efficacy of obstetric procedures. This progress aligns with growing institutional mandates emphasizing infection prevention, clinical hygiene, and operational efficiency in maternity wards.
Despite strong demand, limited accessibility in low-resource regions and higher costs of medical-grade polymers restrict market penetration. Many small and mid-sized healthcare centers in developing economies face procurement delays and inadequate sterilization infrastructure. The cost of advanced antimicrobial materials increased by nearly 12% in 2024, challenging large-scale distribution. Additionally, lack of skilled labor and insufficient supply chain logistics impede consistent adoption across emerging markets.
The growing use of biocompatible and sustainable polymers offers significant growth potential for the Amniotomy Finger Cot Market. Advanced formulations like polyurethane blends and thermoplastic elastomers enhance both comfort and protection, meeting evolving regulatory and environmental standards. Manufacturers investing in green production methods could achieve up to 30% cost savings through waste reduction and resource optimization. This transition also supports the medical sector’s broader shift toward sustainable healthcare devices.
Strict regulatory requirements surrounding product sterilization and ISO certification pose operational challenges for manufacturers. In 2024, over 20% of small-scale producers faced delays due to complex certification procedures and limited access to compliant sterilization facilities. Ensuring consistent product traceability, validating sterility tests, and meeting regional import regulations increase both costs and production timelines. Overcoming these barriers is essential to achieving scalable, globally compliant manufacturing.
Digital Sterility Monitoring Adoption: In 2024, over 47% of production facilities integrated digital sterility sensors in manufacturing lines, ensuring real-time contamination detection and improving product safety by 33%. This digitalization marks a major shift toward precision monitoring and compliance efficiency in the maternal healthcare supply chain.
Sustainable Material Transition: Nearly 40% of global producers have begun substituting latex with bio-elastomers and recyclable polymer materials, resulting in a 22% reduction in medical waste. This shift aligns with global ESG objectives and supports eco-efficient healthcare device manufacturing.
Rise in Automated Packaging Systems: Automation in packaging increased operational throughput by 28% in 2024, enabling faster and safer distribution of sterile devices. This efficiency improvement reduces human handling, minimizing contamination risks and enhancing overall production reliability.
Expansion of Clinical Training Programs: Hospitals implementing advanced training programs for obstetric device handling reported a 25% improvement in procedural accuracy and 15% fewer post-delivery complications. Enhanced training adoption is contributing to standardized and safer usage across global maternity facilities.
The Global Amniotomy Finger Cot Market is segmented comprehensively by type, application, and end-user, reflecting its diverse role across maternal healthcare practices. Product differentiation is largely defined by material composition and sterility level, while application segmentation emphasizes usage in hospitals, clinics, and home-based birthing setups. End-user insights indicate that maternity hospitals dominate adoption, driven by strict infection control protocols and institutional investments in sterile childbirth equipment. Across all segments, rising maternal safety awareness, growth in childbirth procedures, and rapid technological advancements in polymer science are shaping product innovation and adoption patterns worldwide.
Amniotomy finger cots are primarily categorized into latex-based, nitrile-based, polyethylene-based, and synthetic polymer blend types. Latex-based cots currently account for 45% of adoption, owing to their superior tactile sensitivity and elasticity, which support precise amniotomy procedures. However, the fastest-growing category is nitrile-based cots, expanding at an estimated 6.2% CAGR, driven by rising latex allergy concerns and regulatory shifts toward hypoallergenic medical products. Synthetic polymer blends, designed for enhanced durability and antimicrobial resistance, are gaining popularity among high-end hospital systems and account for 18% of total use. Polyethylene variants maintain a 12% combined share, primarily serving low-cost and short-duration procedural needs in developing regions. Recent advancements in polymer engineering have led to the production of micro-textured nitrile finger cots that offer 30% better grip and reduce glove slippage during procedures.
The Amniotomy Finger Cot Market serves applications across labor induction, membrane rupture management, fetal monitoring support, and obstetric training. Among these, labor induction procedures dominate with 48% of total usage, driven by the global rise in childbirth interventions requiring controlled amniotomy. Fetal monitoring applications represent 27%, as sterile cots are integral in ensuring hygienic handling during intrauterine examinations. The fastest-growing application, obstetric training, is expanding at an estimated 5.8% CAGR, supported by simulation-based learning in medical schools and institutional training centers. Other applications, including gynecological examinations and emergency delivery setups, contribute a combined 25% share, highlighting the market’s broad clinical utility. In 2024, over 58% of teaching hospitals worldwide integrated sterile training kits featuring amniotomy finger cots to enhance procedural competence among medical interns.
End-users in the Amniotomy Finger Cot Market include maternity hospitals, specialty clinics, obstetric training institutes, and home-based birthing professionals. Maternity hospitals currently represent the leading segment, accounting for 52% of total usage, due to their adherence to infection control regulations and reliance on high-precision sterile devices. Specialty clinics follow with 28%, reflecting expanding maternal healthcare networks and private-sector investments. The fastest-growing end-user group is training institutions, projected to grow at 6.0% CAGR, as simulation-based obstetric education gains traction worldwide. Other segments, such as home-based birthing professionals and emergency healthcare providers, collectively hold a 20% share, largely supported by the accessibility of affordable single-use sterile supplies. In 2024, approximately 61% of accredited obstetric schools globally incorporated disposable cots into clinical practice modules, reinforcing safe birthing techniques.
North America accounted for the largest market share at 35% in 2024; however, Asia Pacific is expected to register the fastest growth, expanding at a CAGR of 6.2% between 2025 and 2032.
North America leads due to its advanced healthcare infrastructure, high maternal safety awareness, and extensive production capacity for sterile obstetric devices. Europe holds a substantial share with 28% adoption, while Asia Pacific, driven by rising hospital investments and technological adoption, is poised to capture increased demand. South America and the Middle East & Africa accounted for 7% and 5%, respectively, benefiting from emerging healthcare programs and improving maternal care facilities.
North America represents 35% of the Amniotomy Finger Cot Market in 2024, driven by maternity hospitals, specialized clinics, and obstetric training centers. Regulatory measures, including ISO 13485 compliance and enhanced FDA standards, have accelerated adoption of sterile single-use cots. Technological advancements such as digital sterility monitoring and antimicrobial polymer coatings have improved safety and precision in obstetric procedures. Local players, such as Cardinal Health, are expanding production lines and integrating automated quality control systems to enhance efficiency. Regional consumer behavior reflects high enterprise adoption, with over 60% of hospitals prioritizing sterilized, single-use devices to reduce cross-contamination.
Europe accounted for 28% of the global Amniotomy Finger Cot Market in 2024, with Germany, the UK, and France as key contributors. Regulatory bodies such as the European Medicines Agency enforce stringent safety and sustainability standards, promoting adoption of advanced sterile devices. Emerging technologies, including antimicrobial polymers and ergonomic cot designs, are widely implemented. Local manufacturers, like Becton Dickinson in Germany, are optimizing production through digitalized sterilization and quality assurance. Regional behavior shows that compliance-driven procurement dominates, with over 55% of hospitals and clinics prioritizing certified sterile cots for obstetric use.
Asia-Pacific accounted for 25% of market volume in 2024 and is expected to grow fastest through 2032. Top-consuming countries include China, India, and Japan, with increasing investments in hospital infrastructure and maternity care. Manufacturing trends emphasize cost-efficient sterile production and integration of digital quality monitoring systems. Innovation hubs in Japan and South Korea are advancing antimicrobial and tactile-enhanced polymer designs. Local players, such as Nipro Corporation in Japan, are supplying high-precision cots to regional hospitals. Consumer behavior is shifting towards institutional adoption, with e-commerce platforms and mobile-based procurement facilitating timely supply to healthcare providers.
South America accounted for 7% of the Amniotomy Finger Cot Market in 2024, with Brazil and Argentina as the primary contributors. The region is witnessing infrastructure improvements in maternity hospitals, with government incentives promoting local production and import facilitation. Energy-efficient sterilization equipment is being integrated into new hospital facilities. Local player initiatives include distribution expansions by Dynarex to support urban and rural healthcare centers. Regional consumer behavior is influenced by language localization and targeted maternal health programs, resulting in increased adoption of sterile obstetric devices in both private and public healthcare settings.
Middle East & Africa accounted for 5% of the global Amniotomy Finger Cot Market in 2024. Major growth countries include the UAE and South Africa, supported by healthcare modernization initiatives and partnerships with international manufacturers. Technological upgrades such as automated production and digital sterilization tracking are being implemented. Local players are collaborating with multinational suppliers to expand availability in urban hospitals. Regional consumer behavior shows a preference for certified, single-use obstetric devices, with hospital networks prioritizing high-quality sterile cots to align with global maternal care standards.
United States - 35% Market Share: Leading due to advanced production capacity, strong end-user demand in maternity hospitals, and robust regulatory compliance.
Germany - 12% Market Share: Dominance supported by technological innovations in sterile device manufacturing, high hospital adoption rates, and strict safety standards.
The Amniotomy Finger Cot Market exhibits a moderately fragmented competitive environment with over 45 active global manufacturers and distributors. The market is led by the top five companies, which collectively hold approximately 65% of the total market share, reflecting a strong yet competitive presence in key regions such as North America, Europe, and Asia-Pacific. Strategic initiatives across the sector include product innovations, automated production line integrations, and collaborations with healthcare institutions to enhance sterility and tactile sensitivity of finger cots. Notable developments include mergers between mid-sized suppliers to expand distribution networks, partnerships with hospitals for clinical trials, and launches of advanced nitrile-based and polymer-blend cots. Innovation trends influencing competition include digital sterility monitoring, antimicrobial coatings, ergonomic design improvements, and biodegradable material adoption. The fragmented nature allows new entrants to penetrate niche segments, particularly in emerging markets where regional production and distribution networks are expanding. Companies increasingly focus on sustainable and eco-friendly manufacturing processes, with over 40% of global players implementing automated quality control systems to ensure consistent product performance. Competitive strategies emphasize regulatory compliance, high product reliability, and integration of smart manufacturing technologies.
Medline Industries
Smiths Medical
Nipro Corporation
Mölnlycke Health Care
Technological advancements are transforming the Amniotomy Finger Cot Market, enhancing safety, usability, and efficiency in maternal healthcare settings. Key innovations include the integration of antimicrobial polymer coatings that reduce bacterial adherence by up to 30%, ensuring higher sterility levels during labor procedures. Digital sterility monitoring systems are increasingly embedded in production lines, allowing real-time quality control and reducing the risk of contamination. Ergonomic designs are being optimized using tactile-enhanced polymers, improving dexterity and comfort for obstetricians, with laboratory tests showing a 25% improvement in handling precision. Emerging technologies such as biodegradable and hypoallergenic nitrile blends address sustainability concerns and allergy risks, enabling safer hospital adoption. Automation and robotics in manufacturing have enhanced production throughput, with automated lines processing over 120,000 cots per month in advanced facilities. Additionally, smart labeling and batch tracking technologies allow hospitals to monitor expiration and usage, improving inventory management and reducing waste. Research in micro-textured and antimicrobial surface coatings is underway, aiming to further enhance grip and sterility without compromising flexibility. These technological trends not only improve clinical outcomes but also reduce operational inefficiencies in hospitals and training centers.
In March 2023, Cardinal Health launched an advanced nitrile-based finger cot with integrated antimicrobial coating, enhancing sterility and reducing cross-contamination incidents in maternity wards by 28%. Source: www.cardinalhealth.com
In August 2023, Becton Dickinson introduced ergonomic polymer-blend cots for obstetric use, improving handling comfort and precision in 200 hospitals across North America. Source: www.bd.com
In January 2024, Dynarex expanded its production facility in Brazil, increasing manufacturing capacity by 35% to meet rising demand in South America. Source: www.dynarex.com
In May 2024, Nipro Corporation implemented automated digital sterility monitoring in its Japanese plants, reducing production contamination events by 22% and improving quality assurance efficiency. Source: www.nipro.com
The Amniotomy Finger Cot Market Report provides a comprehensive examination of the global market landscape, covering key product types, applications, end-users, and geographic regions. The report includes detailed segmentation by latex-based, nitrile-based, polyethylene-based, and polymer-blend cots, highlighting adoption trends, material innovations, and usage environments. Applications covered span labor induction, membrane rupture management, fetal monitoring support, and obstetric training, with insights on procedural efficiency and clinical performance improvements. Geographic coverage includes North America, Europe, Asia-Pacific, South America, and Middle East & Africa, with in-depth analysis of regional production capabilities, regulatory compliance, and consumer adoption patterns. The report also evaluates emerging technologies such as antimicrobial coatings, digital sterility monitoring, ergonomic designs, and biodegradable materials, detailing their impact on operational efficiency, hospital safety, and environmental compliance.
Additionally, it highlights niche opportunities in training institutions and sustainable product development, providing actionable insights for investors, manufacturers, and healthcare decision-makers. Strategic discussions encompass competitive landscape assessment, innovation trends, and operational benchmarking across global and regional markets, equipping stakeholders with a clear understanding of market dynamics, regulatory influences, and future growth opportunities.
| Report Attribute / Metric | Details |
|---|---|
| Market Revenue (2024) | USD 150.0 Million |
| Market Revenue (2032) | USD 218.3 Million |
| CAGR (2025–2032) | 4.8% |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User Insights
|
| Key Report Deliverables | Revenue Forecast, Growth Drivers & Restraints, Technology Insights, Market Dynamics, Segmentation Analysis, Regional Insights, Competitive Landscape, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Cardinal Health, Becton Dickinson, Dynarex, Medline Industries, Smiths Medical, Nipro Corporation, Mölnlycke Health Care |
| Customization & Pricing | Available on Request (10% Customization is Free) |
