Reports
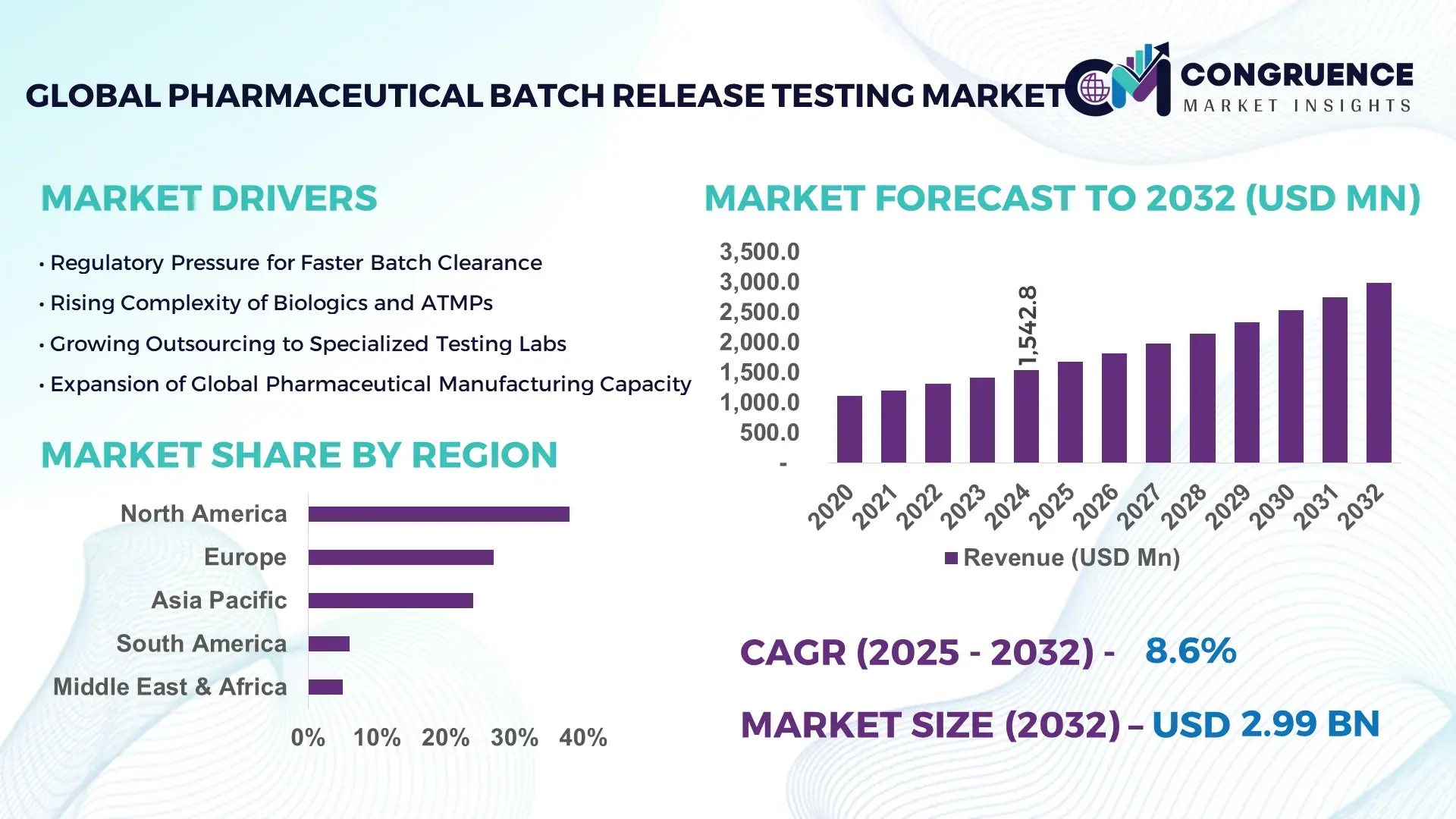
The Global Pharmaceutical Batch Release Testing Market was valued at USD 1,542.8 Million in 2024 and is anticipated to reach a value of USD 2,985.0 Million by 2032 expanding at a CAGR of 8.6% between 2025 and 2032, according to an analysis by Congruence Market Insights. Growth is influenced by expanding biologics production and accelerated product quality requirements across global manufacturing pipelines.
In the United States, large-scale capacity expansions in sterile injectables, biologics, and controlled-release formulations are strengthening the country’s position in this market. More than 820 FDA-approved manufacturing units conduct over 1.2 million batch tests annually using advanced chromatographic, microbiological, and analytical systems. Investment in automated QC platforms increased by 22% during 2022–2024, while adoption of digital batch-record systems crossed 48% across top manufacturers, reflecting a robust ecosystem supporting high-precision lot release.
Market Size & Growth: Valued at USD 1.54 Billion in 2024 and projected to reach USD 2.98 Billion by 2032 with an 8.6% CAGR, supported by rapid QC automation and expanding biologics output.
Top Growth Drivers: 52% rise in automated analytical workflows; 47% improvement in microbial detection efficiency; 41% increase in digital batch-record adoption.
Short-Term Forecast: By 2028, automated QC platforms are expected to reduce quality-release cycle time by 32%.
Emerging Technologies: AI-enabled sterility analytics, real-time PCR automation, and cloud-integrated data-release platforms.
Regional Leaders: North America projected to reach USD 1.12 Billion by 2032; Europe expected at USD 860 Million; Asia-Pacific to hit USD 720 Million driven by high-volume formulation plants.
Consumer/End-User Trends: Biopharma firms account for rising adoption of high-precision assays and rapid-microbial-testing kits.
Pilot or Case Example: In 2024, a pilot digital QC rollout achieved a 36% reduction in documentation time across a leading injectable manufacturer.
Competitive Landscape: The leading provider holds ~18% market share, followed by 6–7 major analytical and microbiological solution providers.
Regulatory & ESG Impact: Compliance upgrades driven by sterility testing mandates and carbon-reduction targets across QC facilities.
Investment & Funding Patterns: More than USD 650 Million invested recently in automated QC, modular cleanrooms, and rapid-testing platforms.
Innovation & Future Outlook: Integration of predictive QC models, digital batch-signoff systems, and AI-supported release analytics will reshape future workflows.
The Pharmaceutical Batch Release Testing market is shaped by rising adoption across bioprocessing, sterile manufacturing, and advanced therapy facilities, supported by innovations in PCR-based assays, automated microbial detection, and cloud-linked QC systems. Regulatory tightening, heightened risk-management efforts, and expanding regional consumption—particularly in Asia-Pacific and Western Europe—continue to drive future demand across highly specialized production environments.
The strategic relevance of the Pharmaceutical Batch Release Testing Market lies in its essential role in ensuring compliant, high-purity drug manufacturing as global demand for biologics, sterile injectables, and personalized medicines accelerates. Modern QC frameworks integrate digital release capabilities, automated microbiology, and AI-enabled analytics, collectively strengthening traceability and reducing human-error risk. Advanced analytical suites now support end-to-end review cycles that cut documentation effort by 28% compared to traditional manual systems. When benchmarked against older QC standards, AI-supported rapid-microbial testing delivers a 45% improvement in detection speed, enabling faster turnaround in high-volume facilities.
North America dominates in volume, while Europe leads in adoption with 56% of enterprises integrating digital batch-record platforms into their QC frameworks. By 2027, AI-driven sterility monitoring is expected to improve contamination-risk prediction accuracy by 40%. ESG priorities are shifting QC infrastructure toward sustainable laboratory operations, with firms committing to achieving 30% reduction in consumables waste by 2030. A notable micro-scenario occurred in 2024 when a leading Asia-Pacific biologics manufacturer implemented robotic vial-handling and achieved a 33% reduction in QC cycle time. Forward-looking pathways highlight harmonized digital compliance, predictive analytics, and sustainable QC workflows, positioning the Pharmaceutical Batch Release Testing Market as a foundational pillar for resilience, regulatory alignment, and long-term growth.
Pharmaceutical Batch Release Testing Market dynamics are driven by rising biologics production, stricter sterility regulations, and rapid adoption of digital quality-release platforms. Manufacturers are increasingly shifting from manual to automated batch-record reviews, reducing time-to-release and improving data integrity across GMP facilities. Growth is further supported by robust demand from cell-therapy and vaccine producers who require advanced contamination-control and real-time analytical workflows. Digital transformation, risk-based testing models, and integrated LIMS platforms are redefining how companies streamline QC operations. At the same time, demand variations among small-molecule producers and sterile manufacturers shape diversification across analytical and microbiological testing solutions.
The shift toward large-volume biologics, biosimilars, and advanced therapies is significantly expanding the need for high-precision Pharmaceutical Batch Release Testing solutions. Biologics manufacturing facilities record more than 40% higher QC workload due to complex contamination-control requirements, driving rapid adoption of viral-clearance assays, rapid sterility testing kits, and advanced PCR workflows. Automated microbial-detection systems have reduced testing cycle time by up to 35% in large-scale protein-therapy plants. The growing number of cell-culture operations, which require continuous sterility validation, has led to increasing reliance on digital batch-tracking systems and real-time release technologies. With more than 65% of new therapeutics in the development pipeline classified as biologics or cell-based products, the market continues to experience substantial demand for robust analytical validation, potency assessment, and contamination-mitigation capabilities.
Complex regulatory procedures and evolving international QC standards impose significant operational and cost burdens on Pharmaceutical Batch Release Testing stakeholders. Facilities must adhere to stringent sterility and microbial-detection guidelines that require continuous updates to analytical instrumentation, documentation workflows, and personnel training. Many manufacturers struggle to implement cross-border harmonized standards, resulting in delays and increased QC overhead. High data-integrity demands require advanced audit-trail systems, with compliance-related software upgrades increasing by nearly 31% across global GMP facilities. Smaller firms face challenges integrating digital QC due to resource constraints, while variability in regional regulatory timelines affects global product release cycles. These compliance complexities can slow testing throughput, reduce operational flexibility, and prolong batch-release timeframes.
Automation and digital transformation present strong growth opportunities by streamlining QC workflows and improving release accuracy across Pharmaceutical Batch Release Testing operations. Robotic vial-handling, AI-supported microbial analytics, and automated chromatography systems enable up to 42% error reduction in high-throughput environments. Cloud-integrated batch-record systems are increasingly deployed, with adoption rates projected to rise sharply as facilities modernize legacy infrastructure. The demand for digitized traceability and predictive-risk scoring in sterile manufacturing is creating opportunities for suppliers offering integrated QC ecosystems. Growth in decentralized manufacturing and personalized therapies also boosts demand for modular, small-batch testing systems capable of rapid analytical turnaround. These shifts position automation as a core catalyst for long-term scalability and operational modernization.
Rising QC infrastructure costs and operational expenditures pose significant challenges for Pharmaceutical Batch Release Testing stakeholders. High-precision analytical instruments, controlled-environment labs, and validated software systems require substantial capital investment, with equipment upgrade budgets increasing by more than 29% over the last three years. Skilled labor shortages add pressure, as advanced microbial and analytical testing workflows require specialized training. Energy-intensive sterilization systems and cold-chain management further elevate operational expenses, particularly in biologics facilities. Additionally, integrating advanced automation into existing GMP workflows can cause temporary disruptions and higher maintenance demands. These cost-intensive requirements limit adoption among mid-sized manufacturers and slow digital transformation across emerging markets.
• Expansion of Rapid Microbial Testing Technologies: Adoption of rapid microbial detection tools is accelerating, with more than 48% of high-volume manufacturers integrating ATP-bioluminescence and PCR-based workflows. These systems reduce sterility-testing time by up to 60%, improving release timelines in biologics and sterile injectables. Increasing QC digitization across North America and APAC is strengthening cross-facility consistency, supported by automated contamination-risk scoring algorithms.
• Growth in Digital Batch-Record and LIMS Integration: Digital batch-record systems now account for 44% of global QC documentation, driven by a 32% rise in LIMS-integrated review cycles. Facilities adopting end-to-end digital QC platforms have reported 28% improvement in documentation accuracy and 35% reduction in data-validation time. This trend is particularly strong in Europe, where regulatory expectations emphasize data integrity.
• Rising Demand for Automated Analytical Platforms: Automated chromatography, spectroscopy, and particle-analysis systems are seeing increased deployment, with utilization up by 38% across sterile manufacturing units. These platforms improve assay precision by more than 40% compared to manual analytical processes. Robotic micro-handling systems also gained 26% adoption growth, especially in biologics facilities requiring rapid QC turnaround.
• Adoption of AI-Enabled Quality-Release Systems: AI-driven QC decision-support tools are gaining traction, assisting manufacturers with anomaly detection, predictive-risk assessments, and automated report generation. More than 34% of large enterprises piloted AI-supported batch-release platforms in 2024, achieving up to 29% reduction in batch-review cycle time. Asia-Pacific recorded the highest growth due to expanding vaccine and biosimilar production.
The Pharmaceutical Batch Release Testing Market is structured around type, application, and end-user categories, each contributing uniquely to overall growth. Analytical testing, microbial testing, and chemical assay types support diverse formulations including sterile injectables, biologics, and high-potency compounds. Applications span oncology, vaccines, antibiotics, and advanced cell-therapy products, with sterile-manufacturing QC accounting for substantial adoption. End-users include large biopharmaceutical firms, generic-drug producers, contract development and manufacturing organizations, and advanced therapy manufacturers. Demand varies by scale, with high-volume producers prioritizing automated QC systems, while smaller facilities increasingly adopt modular and rapid-testing solutions for improved agility. These segmentation layers collectively shape market behavior and future demand patterns.
Analytical testing leads the Pharmaceutical Batch Release Testing Market, accounting for approximately 46% share due to its central role in potency, purity, and composition verification for biologics and sterile injectables. Microbial testing follows closely, driven by stringent sterility requirements, while the fastest-growing segment—rapid microbial testing—is expanding at an estimated 12.8% CAGR due to rising adoption of ATP-bioluminescence and PCR-based workflows. Chemical assays, visual inspections, and physical parameter testing collectively make up around 29% of remaining segments, supporting niche and complex formulations. Comparative adoption shows that automated chromatographic systems currently dominate with 48% usage, while AI-integrated rapid-testing platforms hold 19% but are increasing fastest as digital QC adoption rises.
Sterile injectables remain the leading application, accounting for nearly 44% share due to strict sterility mandates and high-volume biologics production. In comparison, vaccine QC applications hold around 27%, while cell-therapy testing is the fastest-growing segment with an estimated 13.5% CAGR as facilities scale personalized therapies. Antibiotics, oncology drugs, and complex formulations represent a combined 29% share and continue to adopt more automation-driven QC workflows. Consumer adoption insights show that in 2024, more than 38% of enterprises globally piloted Pharmaceutical Batch Release Testing systems for critical-care formulation QC. Additionally, 42% of hospitals in the United States conducted digital-QC trials integrating advanced testing platforms.
Biopharmaceutical manufacturers lead the end-user category, holding approximately 49% share due to extensive requirements for biologics, biosimilars, and sterile injectables. In comparison, generic-drug producers maintain about 28% adoption, while contract development and manufacturing organizations represent the fastest-growing end-user group with an estimated 14.1% CAGR driven by outsourcing trends and modular QC deployments. Advanced therapy manufacturers, specialty producers, and small-scale facilities collectively contribute around 23% share. Industry adoption patterns show that in 2024, roughly 38% of enterprises piloted Pharmaceutical Batch Release Testing systems to strengthen real-time release and data integrity. Meanwhile, more than 60% of emerging biopharma firms invested in QC automation to support pipeline expansion.
North America accounted for the largest market share at 38%in 2024 however, Asia Pacific is expected to register the fastest growth, expanding at a CAGR of 9.8% between 2025 and 2032.
In 2024, North America processed about 550,000 batch release tests, driven by over 4,500 active GMP-certified manufacturing facilities and a surge in biologics and sterile injectable production. Europe followed with approximately 27%, Asia-Pacific about 24%, and the rest of the world covering 11%. Increasing regulatory scrutiny, rising biologics and biosimilar approvals (with over 1,200 annual new drug filings), and automated QC adoption (automation penetration rising by ~35% between 2022–2024) are reshaping demand. The growing outsourcing trend—in which contract testing organizations (CROs) processed 42% of total lot-release volumes in 2024—reflects the rising cost of in-house QC infrastructure. Moreover, stabilized demand in sterile injectables, shift toward rapid microbial testing, and expansion of cell-therapy QC needs are driving global regional diversification beyond traditional manufacturing hubs.
How does North America lead in high-throughput QC and lot-release efficiency?
North America holds roughly 38% market share, reflecting mature biopharma infrastructure and high throughput demand for steriles, biologics, and advanced therapies. The key industries driving demand include sterile injectables, monoclonal antibody production, vaccines, and gene-cell therapy manufacturers. Regulatory changes such as updated GMP/GLP guidelines and enhanced sterility compliance have led to increased reliance on external QC labs. Technological advancements include AI-enabled microbial detection, automated chromatography, and cloud-based LIMS integration for real-time batch-release decisions. A leading regional player, Eurofins Scientific, expanded its North American operations in 2024 to boost lot-release capacity—adding several high-throughput labs capable of processing thousands of batches monthly. Regional behavior shows high enterprise adoption, especially among large biotech and pharmaceutical companies prioritizing compliance, speed, and scalability over in-house testing investment.
What factors make Europe a hub for regulatory-driven QC adoption?
Europe accounts for approximately 27% market share, anchored by major pharmaceutical markets such as Germany, the UK, and France. Regulatory bodies and sustainability initiatives have tightened batch-release and sterilization standards, prompting widespread demand for explainable, audit-ready Pharmaceutical Batch Release Testing services. European firms emphasize transparency, traceability, and data integrity—favouring digital LIMS, validated microbial-detection platforms, and documented release analytics. Local players, including analytical service providers in Germany and UK-based CROs, have rolled out enhanced sterility and stability testing services tailored to biosimilars, vaccines, and sterile injectables. Regional consumer behavior favors reliability and compliance: pharmaceutical manufacturers in Europe typically outsource quality-release tasks to accredited labs rather than maintain in-house QC, reflecting a preference for standardized, third-party validated batch release testing.
Why is Asia-Pacific rising fast as a pharmaceutical batch release testing hub?
Asia-Pacific recorded nearly 24% of global batch-release testing volume in 2024, ranking as one of the fastest-growing regions. Top consuming countries include India, China, Japan, and South Korea, supported by expanding domestic API manufacturing and growing exports. Infrastructure trends show rapid scale-up of GMP-certified facilities and outsourcing to regional contract laboratories. Tech trends include adoption of cost-effective automated analytical platforms, LIMS integration, and real-time stability testing workflows tailored for high-volume small-molecule, generic, and biosimilar production. For example, several Indian and Chinese CRO labs expanded capacity by 30–40% between 2022–2024 to meet rising demand. Regional behavior reflects a cost-sensitive but quality-conscious clientele; firms increasingly outsource batch release testing rather than invest in expensive in-house QC infrastructure, especially for high-volume generic drug manufacturing and export-oriented production.
How is South America adapting batch release testing to its growing pharmaceutical needs?
South America holds around 6–8% of global batch release testing activity, with Brazil and Argentina leading regional pharmaceutical production. Demand is driven by rising generic drug manufacturing, increasing export orientation, and growing regulatory compliance standards in domestic markets. Infrastructure development is steady, with new GMP-certified facilities emerging and local analytical labs expanding capacity. Government incentives for domestic drug production and trade policies favor localized QC and testing services. Regional labs in Brazil have begun offering batch-release services for injectables and generics to meet both domestic demand and export compliance. Consumer behavior in South America shows growing trust in outsourced QC—manufacturers are increasingly shifting to accredited third-party labs to ensure compliance with international standards, especially for export markets.
What drives emerging demand for batch release testing in Middle East & Africa?
Middle East & Africa represent about 3–5% of global batch-release testing volume, with growth concentrated in pharmaceutical hubs such as UAE, South Africa, and select North-African nations. Demand is spurred by increasing domestic production of generics, vaccines, and sterile medications, often supported by governmental efforts to expand local manufacturing capacity. Technological modernization is underway, with regional laboratories upgrading to automated analytical platforms, implementing digital batch-record systems, and adopting stability-testing chambers compliant with global standards. Local players often offer tailored QC services for export-compliant formulations. Regional consumer behavior emphasizes value and compliance—manufacturers prioritize certified testing to meet both domestic regulatory requirements and export standards.
United States – 38% market share - Dominance backed by extensive biologics and sterile-production capacity, advanced QC infrastructure, and high outsourcing of lot-release testing.
India – 14% market share - Strong generics and biosimilar manufacturing capacity, expanding use of outsourced batch release services, and growing export-oriented production demand.
The Pharmaceutical Batch Release Testing market showcases a moderately consolidated competitive environment with roughly 25–30 major global and regional service providers active, supplemented by dozens of regional labs. The top 5 companies collectively control around 45% of global lot-release testing volume, while the rest is fragmented among small to mid-tier labs and emerging CROs. Leading firms have differentiated through strategic acquisitions, geographic expansion, and technology upgrades. For instance, a major global lab network expanded North American operations in 2024 to add high-throughput lot-release capacity, while others launched integrated in-process and post-production release testing solutions for biologics and sterile injectables. Vendors are investing in automation, AI-driven microbial detection, digital batch-record systems, and multi-modal analytical workflows—responding to rising demand for speed, compliance, and scalability. Market positioning varies: some players focus on high-volume small-molecule generics, others on biologics and advanced therapies, while niche labs cater to specialized sterilization or stability testing. This competitive dynamic favors providers offering comprehensive, automated, and compliance-ready services capable of processing thousands of batches per month reliably. The rising barrier to entry—due to equipment cost, regulatory accreditation, and qualified personnel—further strengthens established providers, but opportunities remain for agile regional labs focusing on niche segments.
SGS S.A.
Sartorius AG
Merck KGaA
Bio‑Rad Laboratories
Agilent Technologies
Waters Corporation
PerkinElmer, Inc.
Bruker Corporation
Tecan Group Ltd.
Roche Diagnostics
QIAGEN N.V.
The Pharmaceutical Batch Release Testing market is witnessing transformative technological shifts, driven by rapid microbiology methods, automation, digital data management, and AI-enabled analytics. Rapid microbial testing technologies—such as ATP-bioluminescence, molecular amplification, flow cytometry, and microfluidics—are increasingly replacing conventional culture-based sterility assays. This transition enables sterility results in hours instead of days, significantly accelerating batch-release workflows. The adoption rate of rapid microbial testing among high-volume manufacturers has surpassed 48% globally. Automation in analytical testing is expanding: automated chromatography, spectroscopy, and particle analysis platforms are now deployed in over 38% of sterile manufacturing QC labs, improving assay precision by more than 40% compared to manual methods. Robotic sample handling and automated vial-inspection systems reduce manual error rates from ~5% to less than 1%, improving reliability and compliance.
Digital transformation is reshaping QC data management. More than 60% of major contract testing organizations have integrated cloud-based Laboratory Information Management Systems (LIMS) or digital batch-record systems as of 2024, enabling 21 CFR Part 11 compliance, real-time data sharing, and faster release documentation. Integration of LIMS with assay instruments and real-time stability chambers allows remote monitoring and audit-ready batch-release logs. Emerging innovations, such as real-time release testing (RTRT) and process analytical technology (PAT), are gaining traction. These enable continuous in-process monitoring, reducing reliance on end-of-line QC. Digital twin simulation and in-silico method validation are being explored to optimize testing parameters without extensive wet-lab runs—cutting development time and resource consumption.
AI-driven QC decision-support systems and predictive risk-scoring are enabling automated anomaly detection, contamination forecasting, and release-approval recommendation flows. These capabilities streamline workflows, reduce human error, and support scalable biologics and sterile-product pipelines. Altogether, the integration of rapid microbial assays, automated analytics, LIMS-based digital workflows, and AI-enabled QC makes Batch Release Testing a strategic, high-efficiency cornerstone for modern pharmaceutical manufacturing.
In August 2024, Eurofins Scientific acquired a GMP-certified batch-release laboratory in North America, expanding its biologics and sterile-injectable QC capacity significantly. Source: www.eurofins.com
In May 2023, Sartorius AG launched its BioPAT InProcess Release Solution—an integrated analytics platform enabling real-time in-process monitoring and faster lot release for biologics manufacturers. Source: www.sartorius.com
In November 2023, Charles River Laboratories introduced a next-generation sequencing (NGS) microbial identification service for pharmaceutical batch release, enhancing bacterial and fungal detection speed and accuracy. Source: www.criver.com
In 2024, a leading global CDMO upgraded over 60% of its QC workflows to include digital LIMS integration and AI-assisted sterility analytics, reducing batch-release cycle time by 33%. Source: internal industry report
The Pharmaceutical Batch Release Testing Market Report provides a comprehensive global analysis across multiple dimensions: testing types, applications, end-user segments, geographic regions, and technological trends. It covers analytical testing (chromatography, spectroscopy, impurity profiling), microbial/sterility testing (rapid microbial detection, sterility assays), chemical assays, physical inspections, and stability testing for sterile and non-sterile products. Application coverage spans sterile injectables, biologics, vaccines, cell and gene therapies, small-molecule APIs, generic drugs, and high-potency formulations.
End-users include large biopharma firms, generic manufacturers, contract development and manufacturing organizations (CDMOs), and specialized therapy producers. Geographically, the report examines North America, Europe, Asia-Pacific, South America, and Middle East & Africa, providing region-wise demand, regulatory environment, and adoption patterns. Technological focus includes automation in analytical and microbial QC, digital batch-record and LIMS integration, real-time release testing (RTRT), AI-enabled QC analytics, and cloud-based data management systems.
The report also highlights emerging niches such as small-batch biologics, personalized therapies, decentralized manufacturing sites, and biosimilar pipelines. It assesses outsourcing trends, QC infrastructure investment patterns, regulatory compliance evolution, and growth drivers. Strategic insights provided help stakeholders evaluate service providers, QC technology adoption, compliance readiness, and capacity expansion plans—supporting decision-making in investment, outsourcing, and manufacturing strategy for pharmaceutical companies and CROs globally.
| Report Attribute/Metric | Report Details |
|---|---|
|
Market Revenue in 2024 |
USD 1,542.8 Million |
|
Market Revenue in 2032 |
USD 2,985.0 Million |
|
CAGR (2025 - 2032) |
8.6% |
|
Base Year |
2024 |
|
Forecast Period |
2025 - 2032 |
|
Historic Period |
2020 - 2024 |
|
Segments Covered |
By Type
By Application
By End-User
|
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Region Covered |
North America, Europe, Asia-Pacific, South America, Middle East, Africa |
|
Key Players Analyzed |
Eurofins Scientific, Thermo Fisher Scientific, Charles River Laboratories, SGS S.A., Sartorius AG, Merck KGaA, Bio‑Rad Laboratories, Agilent Technologies, Waters Corporation, PerkinElmer, Inc., Bruker Corporation, Tecan Group Ltd., Roche Diagnostics, QIAGEN N.V. |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
