Reports
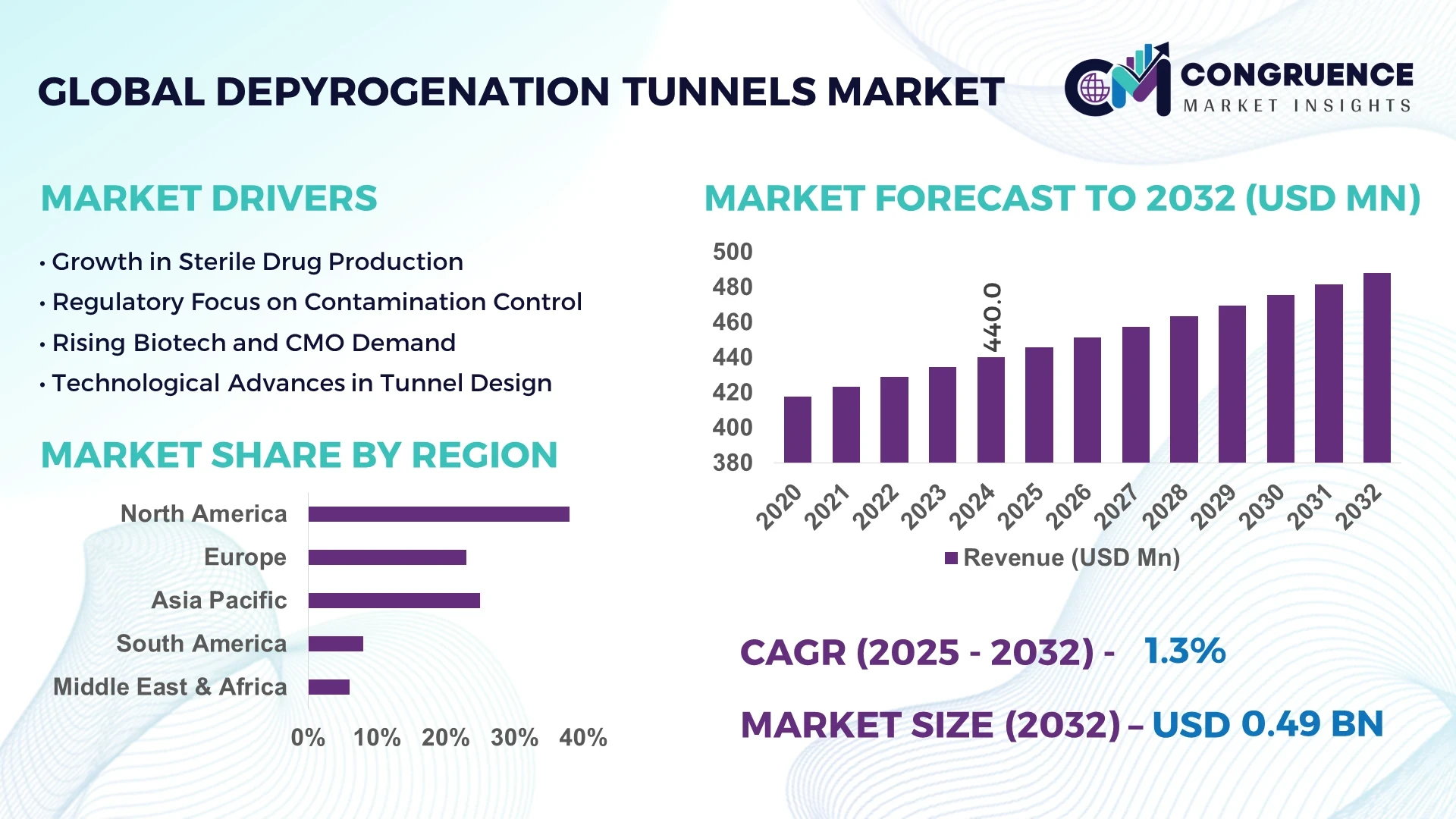
The Global Depyrogenation Tunnels Market was valued at USD 440 Million in 2024 and is anticipated to reach a value of USD 487.9 Million by 2032, expanding at a CAGR of 1.3% between 2025 and 2032.
North America dominates the global depyrogenation tunnels market, primarily due to its well-established pharmaceutical industry and stringent regulatory frameworks. The United States, in particular, has a significant number of pharmaceutical companies and research institutions that require advanced depyrogenation solutions to ensure product safety and compliance. The region's focus on innovation and technological advancements is also driving the demand for sophisticated depyrogenation tunnels.
Depyrogenation tunnels are critical in pharmaceutical manufacturing, ensuring the sterility of glassware and tools by effectively removing pyrogens through controlled heated air circulation. These systems are indispensable in modern pharmaceutical processes, particularly in the production of sterile injectable drugs. The increasing demand for biopharmaceuticals and the rise in chronic diseases necessitate stringent sterilization processes, further propelling the market growth. Technological advancements, such as real-time monitoring and automated control systems, are enhancing the efficiency and reliability of depyrogenation tunnels, making them more attractive to pharmaceutical companies and contract manufacturing organizations.
Artificial Intelligence (AI) is revolutionizing the depyrogenation tunnels market by enhancing efficiency, precision, and compliance in sterilization processes. AI algorithms optimize sterilization cycles by analyzing real-time data, adjusting parameters to achieve maximum efficacy. This level of precision is crucial in settings where contamination risks are high. The integration of AI reduces human error, ensures consistent and repeatable results, and facilitates predictive maintenance, thereby minimizing downtime and operational costs. Moreover, AI-driven systems can monitor the sterilization environment and make immediate adjustments, ensuring that every item is adequately treated. This technological advancement is particularly relevant as healthcare facilities strive to maintain high standards of hygiene in the wake of global pandemics. Regulatory bodies are increasingly focusing on the validation of new sterilization technologies, and compliance with stringent guidelines will ensure that innovations are not only effective but also safe for use in critical applications. The integration of AI into depyrogenation tunnels is expected to propel the industry forward, offering enhanced safety, sustainability, and efficiency in pharmaceutical manufacturing.
“In 2024, Steris Corporation launched a new range of automated depyrogenation ovens with advanced digital monitoring systems, integrating AI to optimize sterilization cycles and enhance operational efficiency.”
The increasing prevalence of chronic diseases and the growing need for sterile injectable drugs are significant drivers of the depyrogenation tunnels market. With an aging global population and heightened healthcare needs, the consumption of injectable drugs is on the rise. These drugs must be free from pyrogens to ensure patient safety, necessitating the use of depyrogenation tunnels. Additionally, stringent regulatory guidelines imposed by health authorities mandate the use of reliable depyrogenation processes, further boosting market demand.
Despite the growth potential, the depyrogenation tunnels market faces challenges due to the high initial investment required for purchasing and maintaining depyrogenation equipment. Small and medium-sized enterprises may find it challenging to allocate the necessary budget for such technologies, which can limit their ability to compete effectively in the market. Furthermore, the complexity of the depyrogenation process requires skilled personnel to operate and maintain these sophisticated systems, leading to increased operational costs.
The integration of automation and advanced technologies in depyrogenation tunnels presents significant opportunities for market growth. AI and machine learning can enhance efficiency, reduce operational costs, and improve product quality. As manufacturers look to optimize their operations, the demand for innovative solutions in this space is expected to rise. Moreover, the increasing focus on sustainability and green manufacturing practices presents opportunities for companies to develop eco-friendly depyrogenation solutions that reduce energy consumption and minimize environmental impact.
The depyrogenation tunnels market faces challenges related to regulatory compliance and validation. Pharmaceutical manufacturers must adhere to stringent standards set by regulatory bodies, which require rigorous validation of sterilization processes. Navigating these regulatory landscapes requires substantial investment in compliance infrastructure and expertise, posing a barrier to market entry for new players. Additionally, the complexity of regulatory compliance can pose challenges for companies, particularly in regions with less developed regulatory frameworks.
Integration of IoT and AI Technologies: The adoption of Internet of Things (IoT) and AI technologies is transforming depyrogenation processes. Smart devices can track sterilization cycles and provide real-time feedback, ensuring compliance with standards and improving overall operational efficiency. This interconnected approach facilitates better decision-making and resource management in healthcare facilities.
Customization for Biopharmaceuticals: With the rise of biopharmaceuticals, there is a trend towards customizing depyrogenation solutions to meet the specific needs and sensitivities of biologics manufacturing. This includes the development of specialized equipment and processes that cater to the unique requirements of biopharmaceutical products.
Validation Automation: Automation in validation processes, including the use of electronic documentation and real-time monitoring, is a trend to streamline compliance requirements. This reduces the time and resources required for validation, enhancing efficiency and ensuring adherence to regulatory standards.
Expansion in Emerging Markets: The need for depyrogenation tunnels is rising as emerging markets in areas like Asia-Pacific and Latin America develop their healthcare infrastructure. Sterilization technology adoption is being driven by the expansion of pharmaceutical and vaccine manufacturing in these markets. The market for depyrogenation tunnels is anticipated to rise significantly due to the growing need for safe and high-quality medicinal goods in these areas.
The global depyrogenation tunnels market is segmented into types, applications, and end-users, each influencing the market’s growth uniquely. By type, the market includes horizontal, vertical, and customized depyrogenation tunnels, with varying adoption based on production scale and spatial needs. Applications span sterilizing vials, ampoules, syringes, cartridges, and other pharmaceutical glassware critical for injectable drugs. End-users primarily consist of pharmaceutical companies, biotechnology firms, and contract manufacturing organizations (CMOs), each with distinct sterilization volume requirements and investment capabilities. These segments highlight how market players tailor their strategies to meet evolving regulatory demands and technological advancements. Regional expansion, increasing sterile drug production, and growing outsourcing trends further shape these segments' growth trajectories.
The depyrogenation tunnels market is primarily divided into horizontal, vertical, and custom-configured tunnels. Horizontal depyrogenation tunnels lead the market, accounting for the largest share in 2024 due to their high throughput capacity and suitability for continuous production processes in large pharmaceutical manufacturing facilities. Their design supports efficient airflow and consistent sterilization, making them ideal for bulk handling of glassware like vials and ampoules.
Meanwhile, vertical depyrogenation tunnels are the fastest-growing segment owing to their space-saving design and lower energy consumption, which appeals to small and medium-scale pharmaceutical and biotech firms with limited floor space. These tunnels are increasingly adopted in modular cleanroom setups, where footprint optimization is crucial.
Additionally, customized tunnels tailored to specific production needs are gaining traction, especially among specialty drug manufacturers focusing on small-batch and personalized medicines. This customization trend supports flexible sterilization workflows aligned with evolving pharmaceutical manufacturing demands.
Application-wise, the market includes vials, ampoules, syringes, cartridges, and others. Vials are the dominant application segment due to their extensive use in injectable drugs, vaccines, and biologics, driving demand for large-scale depyrogenation solutions. The rise in vaccine production and parenteral drug use continues to bolster the vial sterilization segment.
The fastest-growing application is syringes, propelled by increasing adoption of pre-filled syringes and auto-injectors for chronic disease management and immunization programs. Syringe sterilization demands high precision to maintain product integrity, encouraging innovations in depyrogenation tunnel designs.
Ampoules and cartridges maintain steady demand, particularly in niche therapeutic segments where precise sterilization of small-volume glass containers is critical. Overall, the application mix reflects pharmaceutical trends toward injectable and biologic therapies.
The market’s end-users are segmented into pharmaceutical companies, biotechnology firms, contract manufacturing organizations (CMOs), and others including research institutes. Pharmaceutical companies currently hold the largest market share, owing to their extensive production volumes, strict regulatory compliance needs, and investments in high-throughput depyrogenation tunnels.
However, CMOs are the fastest-growing end-user segment, driven by the increasing outsourcing of manufacturing and sterilization services by pharmaceutical and biotech companies. CMOs invest heavily in advanced sterilization technologies to meet diverse client requirements, supporting market expansion.
Biotechnology firms, though smaller scale users, are steadily increasing their demand for flexible, small-batch depyrogenation tunnels suitable for biologics and personalized medicine production, contributing to the diversification and innovation in the market.
North America accounted for the largest market share at 38% in 2024; however, Asia-Pacific is expected to register the fastest growth, expanding at a CAGR of 2.1% between 2025 and 2032.
North America’s dominance is attributed to its mature pharmaceutical industry, strict regulatory frameworks, and high adoption of advanced sterilization technologies. Meanwhile, Asia-Pacific’s rapid industrialization, expanding pharmaceutical manufacturing base, and increased investments in healthcare infrastructure fuel its accelerating market growth. Europe holds a significant share due to stringent hygiene standards and advanced manufacturing technologies, while Latin America and the Middle East & Africa are emerging regions witnessing gradual growth driven by healthcare modernization and increasing awareness about drug safety.
Innovation and Regulatory Stringency Drive Market Expansion
North America’s depyrogenation tunnels market is marked by continuous innovation and the adoption of cutting-edge technologies. The U.S. pharmaceutical sector leads in integrating AI and IoT in sterilization processes to improve efficiency and compliance. Canada contributes through growing biotech research centers demanding sterile processing solutions. The region’s strict FDA guidelines drive investments in high-precision depyrogenation tunnels to ensure product safety. Furthermore, rising demand for vaccines and sterile injectables in both the U.S. and Canada fuels demand. The presence of leading pharmaceutical manufacturers and contract manufacturing organizations further solidifies North America’s position as a market leader.
Sustainability and Automation Shape European Market Growth
Europe’s depyrogenation tunnels market is evolving with a strong focus on sustainability and automation. Germany, the U.K., and France are at the forefront, investing heavily in energy-efficient depyrogenation technologies that reduce carbon footprint. The rise in biosimilar drug production drives demand for highly reliable and automated sterilization tunnels. European manufacturers are also adopting modular and flexible depyrogenation systems to support small-batch biologics and personalized medicines. Regulatory frameworks across the EU emphasize environmental compliance and product safety, motivating companies to upgrade their sterilization processes continuously.
Rapid Industrialization and Healthcare Infrastructure Expansion
Asia-Pacific is experiencing rapid growth in depyrogenation tunnels, driven primarily by expanding pharmaceutical manufacturing hubs in China, India, Japan, and South Korea. Investments in healthcare infrastructure and increasing government support for domestic drug production are accelerating market adoption. China leads with extensive vaccine manufacturing and contract manufacturing services requiring advanced depyrogenation systems. India’s booming generic drug sector also propels demand. Additionally, the rise in biologics and biosimilars production calls for sophisticated sterilization technologies. Countries like Japan focus on innovation and compliance, further contributing to the region’s market momentum.
Emerging Market Growth Amidst Healthcare Modernization
South America’s depyrogenation tunnels market is growing steadily, led by Brazil and Argentina, where pharmaceutical industries are modernizing manufacturing facilities to meet global standards. The increasing demand for sterile injectables and vaccines in the region drives investments in depyrogenation equipment. Brazil’s expanding biotech sector and government initiatives to improve healthcare infrastructure positively impact market growth. Despite slower adoption rates compared to North America and Europe, growing regulatory awareness and local manufacturing capabilities signal strong future potential for advanced depyrogenation tunnel systems in South America.
Healthcare Investment Spurs Gradual Market Growth
The Middle East & Africa region is witnessing gradual growth in the depyrogenation tunnels market due to increased government spending on healthcare infrastructure, especially in the UAE and South Africa. These countries are developing pharmaceutical manufacturing and vaccine production facilities that require sterile processing solutions. The growing focus on meeting international quality standards encourages the adoption of advanced depyrogenation technologies. While the market is still emerging, regional partnerships and investments in biotechnology sectors contribute to increasing demand. Awareness programs about drug safety and hygiene are also promoting market development.
United States: holds the highest market share valued at approximately USD 167 million in 2024, driven by its large pharmaceutical manufacturing base and stringent regulatory environment.
China: follows as the second-largest market with a valuation of around USD 95 million, fueled by rapid industrial growth and expanding vaccine and biologics production capacity.
The global depyrogenation tunnels market is highly competitive, with numerous established players and emerging companies vying for market share through technological innovation and strategic partnerships. Leading manufacturers are focusing on product innovation, expanding production capabilities, and enhancing service offerings to maintain their competitive edge. The market is characterized by the presence of companies investing heavily in automation, AI integration, and environmentally friendly technologies to meet growing regulatory demands and customer expectations. Several players have also entered into collaborations and joint ventures to strengthen their market presence across different regions. With increasing demand for sterile injectable drugs and vaccine production, companies are prioritizing the development of high-throughput and energy-efficient depyrogenation tunnels that reduce operational costs while ensuring product safety and quality.
Getinge AB
Azbil Corporation
Federal Equipment Company
Mediseal Group
Peter Huber Kältemaschinenbau AG
Tenova S.p.A
Stork Technical Services (part of Fluor Corporation)
Nabertherm GmbH
Optima Packaging Group GmbH
STERIS plc
Technology in depyrogenation tunnels has advanced significantly, focusing on improving sterilization efficiency, energy savings, and process automation. Modern tunnels utilize precise temperature control and optimized airflow management to ensure uniform heat distribution for effective pyrogen removal from glassware and pharmaceutical containers. Many systems now integrate real-time monitoring sensors and data logging capabilities, enhancing process validation and compliance with stringent regulatory standards. The incorporation of automation technology, including programmable logic controllers (PLCs) and human-machine interfaces (HMIs), allows for streamlined operations and reduced human error. Additionally, eco-friendly technologies such as energy recovery systems and improved insulation materials are being adopted to reduce energy consumption and carbon footprints. Emerging technologies like AI-based predictive maintenance and IoT connectivity enable proactive troubleshooting and operational efficiency improvements, helping manufacturers reduce downtime and extend equipment life cycles.
In January 2024, Getinge AB launched an advanced depyrogenation tunnel featuring AI-enabled temperature optimization that reduces energy consumption by up to 15% while maintaining sterilization efficacy.
In September 2023, Azbil Corporation introduced a new line of modular depyrogenation tunnels designed for flexible installation in small to medium pharmaceutical manufacturing units, enabling faster setup and customization.
In March 2024, Federal Equipment Company expanded its depyrogenation product portfolio by unveiling a compact tunnel system tailored for biotech firms focusing on personalized medicine production.
In November 2023, Mediseal Group announced the integration of IoT sensors across their depyrogenation tunnel range, facilitating remote monitoring and predictive maintenance to enhance operational reliability.
The depyrogenation tunnels market report covers comprehensive analysis and insights into the global market landscape, focusing on key segments such as type, application, and end-user. It provides detailed regional market assessments, highlighting growth opportunities and challenges across North America, Europe, Asia-Pacific, South America, and the Middle East & Africa. The report examines technological advancements, competitive dynamics, and regulatory influences shaping the market. It also emphasizes recent developments, investment trends, and strategic initiatives by major players. Furthermore, the report offers forecasts and market sizing, aiding stakeholders in decision-making processes. By addressing factors like demand drivers, restraints, and emerging trends, the report serves as a valuable resource for manufacturers, suppliers, investors, and policymakers engaged in the depyrogenation tunnels market ecosystem.
| Report Attribute / Metric | Report Details |
|---|---|
| Market Name | Global Depyrogenation Tunnels Market |
| Market Revenue (2024) | USD 440 Million |
| Market Revenue (2032) | USD 487.9 Million |
| CAGR (2025–2032) | 1.3% |
| Base Year | 2024 |
| Forecast Period | 2025–2032 |
| Historic Period | 2020–2024 |
| Segments Covered |
By Type
By Application
By End-User
|
| Key Report Deliverables | Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape, Technological Insights, Recent Developments |
| Regions Covered | North America, Europe, Asia-Pacific, South America, Middle East & Africa |
| Key Players Analyzed | Getinge AB, Azbil Corporation, Federal Equipment Company, Mediseal Group, Peter Huber Kältemaschinenbau AG, Tenova S.p.A, Stork Technical Services (part of Fluor Corporation), Nabertherm GmbH, Optima Packaging Group GmbH, STERIS plc |
| Customization & Pricing | Available on Request (10% Customization is Free) |
